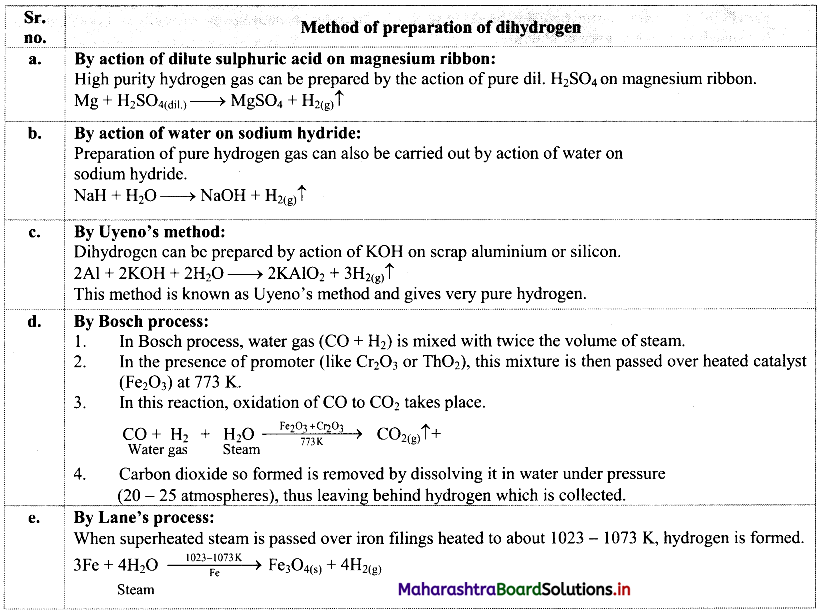
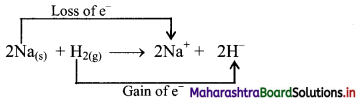
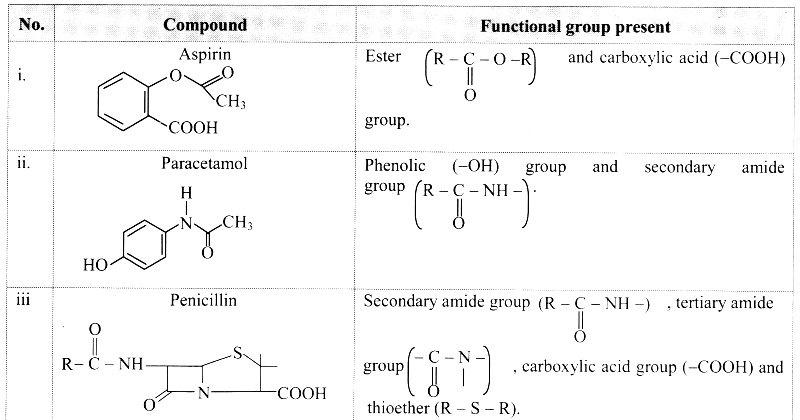
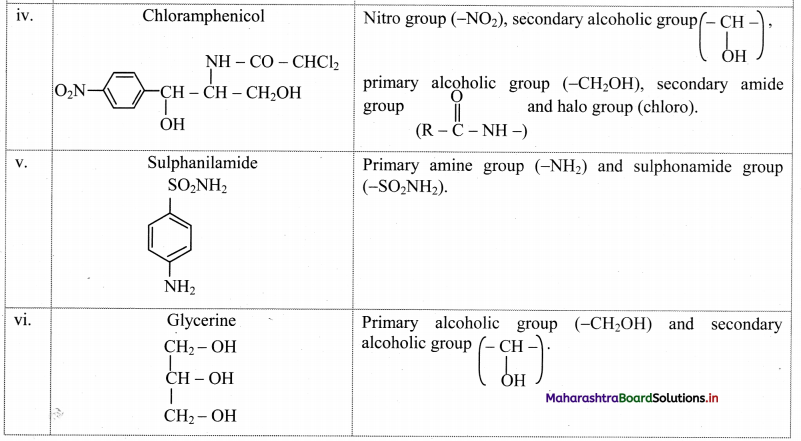
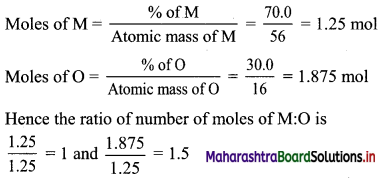
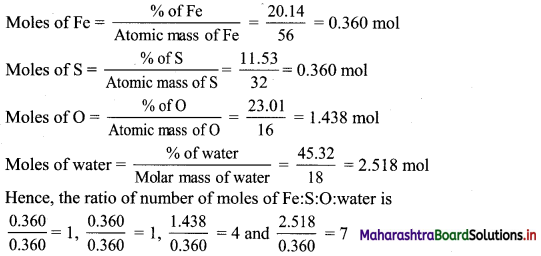
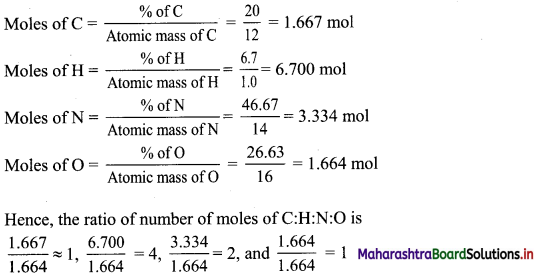
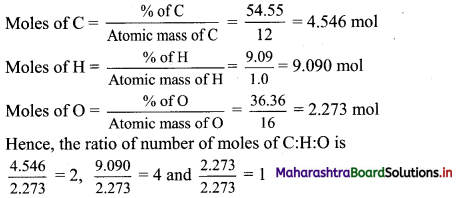
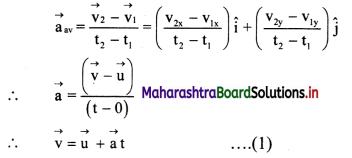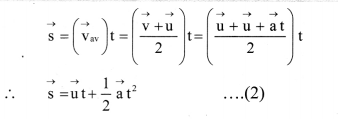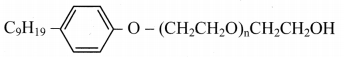Balbharti Maharashtra State Board 11th Biology Textbook Solutions Chapter 14 Human Nutrition Textbook Exercise Questions and Answers.
Human Nutrition Class 11 Exercise Question Answers Solutions Maharashtra Board
Class 11 Biology Chapter 14 Exercise Solutions Maharashtra Board
Biology Class 11 Chapter 14 Exercise Solutions
1. Choose correct option
Question A.
Acinar cells are present in ……………..
a. liver
b. pancreas
c. gastric glands
d. intestinal glands
Answer:
b. pancreas
Question B.
Which type of teeth are maximum in number in human buccal cavity?
a. Incisors
b. Canines
c. Premolars
d. Molars
Answer:
d. Molars
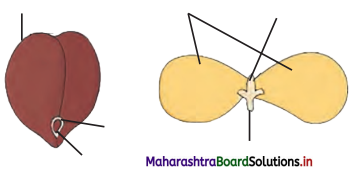
![]()
Question C.
Select odd one out on the basis of digestive functions of tongue.
a. Taste
b. Swallowing
c. Talking
d. Mixing of saliva in food
Answer:
c. Talking
Question D.
Complete the analogy:
Ptyalin: Amylase : : Pepsin : …………….. .
a. Lipase
b. Galactose
c. Proenzyme
d. Protease
Answer:
d. Protease
2. Answer the following questions
Question A.
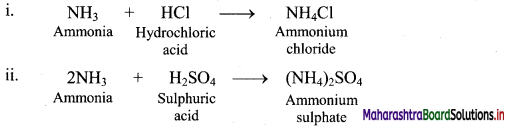
For the school athletic meet, Shriya was advised to consume either Glucon-D or fruit juice but no sugarcane juice. Why it must be so?
Answer:
Sugarcane juice contain disaccharides. Disaccharides take time to digest i.e. breaking into monosaccharides, Glucon — D and fruit juices contain monosaccharide. Therefore, for instant supply of energy during athletic meet Glucon – D or fruit juices are preferred and not sugarcane.
Question B.
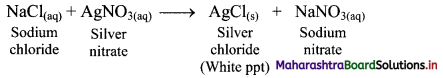
Alcoholic people may suffer from liver disorder. Do you agree? Explain your answer.
Answer:
- Liver disorder in alcoholic people may occur after years of heavy drinking.
- Most of the alcohol in the body is broken down in the liver by an enzyme called alcohol dehydrogenase, which transforms ethanol into a toxic compound called acetaldehyde (CH3CHO).
- ver consumption of alcohol leads to cirrhosis (distorted or scarred liver) and eventually to liver failure.
Therefore, alcoholic people may suffer from liver disorder.
![]()
Question C.
Digestive action of pepsin comes to a stop when food reaches small intestine. Justify.
Answer:
Pepsin acts in acidic medium thus it is active in stomach. There is alkaline condition in the small intestine. pH of small intestine is very high for pepsin to work. Therefore, pepsin gets denatured in the small intestine.
Question D.
Small intestine is very long and coiled. Even if we jump and run, why it does not get twisted? What can happen if it gets twisted?
Answer:
- Mesentery is a tissue that is located in the abdomen. It attaches the small intestine to the wall of the abdomen and keeps it in place and therefore it does not get twisted while running and jumping.
- If small intestine gets twisted, the affected spot may block the food, liquid passing through it. It may sometimes cut off the blood flow if the twist is very severe. If this happens the surrounding tissue may die and can cause serious problems.
3. Write down the explanation
Question A.
Digestive enzymes are secreted at appropriate time in our body. How does it happen?
Answer:
- The digestive enzymes and juices are produced in sequential manner and at a proper time.
- These secretions are under neurohormonal control.
- Sight, smell and even thought of food trigger saliva secretion.
- Tenth cranial nerve stimulates secretion of gastric juice in stomach.
- Even the hormone gastrin brings about the same effect.
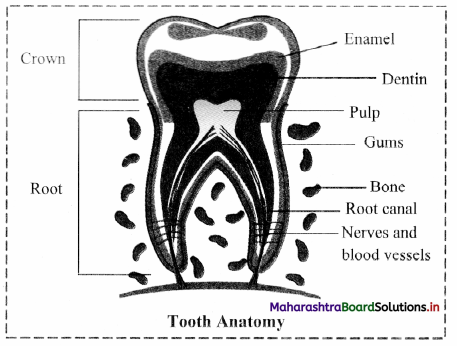
B. Explain the structure of tooth. Explain why human dentition is considered as thecodont, diphydont and heterodont.
Answer:
- Structure of tooth:
- A tooth consists of the portion that projects above the gum called crown and the root that is made up of two or three projections which are embedded in gum.
- A short neck connects the crown with the root.
- The crown is covered by the hardest substance of the body called enamel which is made up of calcium phosphate and calcium carbonate.
- Basic shape of tooth is derived from dentin which is a calcified connective tissue.
- The dentin encloses the pulp cavity. It is filled with connective tissue pulp. It contains blood vessels and nerves.
- Pulp cavity has extension in the root of the tooth called root canal.
- The dentin of the root of tooth is covered by cementurn which is a bone like substance that attaches the root to the surrounding socket in the gum.
- Human dentition is described as thecodont, diphyodont and heterodont.
- It is called the codont type because each tooth is fixed in a separate socket present in the jaw bones by gomphosis type of joint.
- It is called diphyodont type because we get only two sets of teeth, milk teeth and permanent teeth.
- It is called heterodont type because humans have four different type of teeth like incisors, canines, premolars and molars.
![]()
Question C.
Explain heterocrine nature of pancreas with the help of histological structure.
Answer:
Pancreas:
- Pancreas is a leaf shaped heterocrine gland present in the gap formed by bend of duodenum under the stomach.
- Exocrine part of pancreas is made up of acini, the acinar cells secrete alkaline pancreatic juice that contains various digestive enzymes.
- Pancreatic juice is collected and carried to duodenum by pancreatic duct.
- The common bile duct joins pancreatic duct to form hepato-pancreatic duct. It opens into duodenum.
- Opening of hepato-pancreatic duct is guarded by sphincter of Oddi.
- Endocrine part of pancreas is made up of islets of Langerhans situated between the acini.
- It contains three types of cells a-cells which secrete glucagon, P-cells which secretes insulin and 5 cells secrete somatostatin hormone.
- Glucagon and insulin together control the blood-sugar level.
- Somatostatin hormone inhibits glucagon and insulin secretion.
4. Write short note on
Question A.
Position and function of salivary glands.
Answer:
Salivary Glands:
- There are three pairs of salivary glands which open in buccal cavity.
- Parotid glands are present in front of the ear.
- The submandibular glands are present below the lower jaw.
- The glands present below the tongue are called sublingual.
- Salivary glands are made up of two types of cells.
- Serous cells secrete a fluid containing digestive enzyme called salivary amylase.
- Mucous cells produce mucus that lubricates food and helps swallowing.
Question B.
Jaundice
Answer:
- Jaundice is a disorder characterized by yellowness of conjunctiva of eyes and skin and whitish stool.
- It is a sign of abnormal bilirubin metabolism and excretion.
- Jaundice develops if excessive break down of red blood cells takes place along with increased bilirubin level than the liver can handle or there is obstruction in the flow of bile from liver to duodenum.
- Bilirubin produced from breakdown of haemoglobin is either water soluble or fat soluble.
- Fat soluble bilirubin is toxic to brain cells.
- There is no specific treatment to jaundice.
- Supportive care, proper rest are the treatments given to the patient.
[Note: Treatment ofjaundice will depend on the underlying cause of it. For example, hepatitis-induced jaundice would require treatment which includes antiviral or steroid medications ]
![]()
Question 5.
Observe the diagram. This is histological structure of stomach. Identify and comment on significance of the layer marked by arrow.
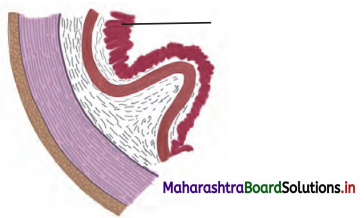
Answer:
The layer marked in the diagram represents glandular epithelium of mucosa.
Significance of the glandular epiihelium of mucosa:
Goblet cells of the epithelial layer of a mucous membrane secrete mucus which lubricates the lumen of the alimentary canal. This helps in movement of food through the gastrointestinal tract.
Question 6.
Find out pH maxima for salivary amylase, trypsin, nucleotidase and pepsin and place on the given pH scale
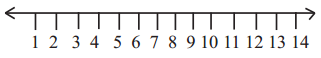
Answer:
Salivary amylase = 6.8
Trypsin = 8
Nucleotidase = 7.5
Pepsin = 2
Question 7.
Write the name of a protein deficiency disorder and write symptoms of it.
Answer:
- Kwashiorkor is a protein deficiency disorder.
- This protein deficiency disorder is found generally in children between one to three years of age.
- Children suffering from Kwashiorkor are underweight and show stunted growth, poor brain development, loss of appetite, anaemia, protruding belly, slender legs, bulging eye, oedema of lower legs and face, change in skin and hair colour.
Question 8.
Observe the diagram given below label the A, B, C, D, E and write the function of A, C in detail.
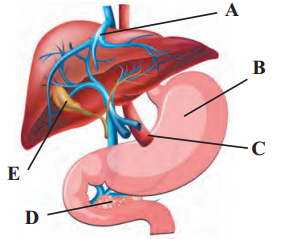
Answer:
A- Bile duct, B- Stomach, C- Common hepatic duct, D- Pancreas, E- Gall Riadder
Functions: Bile duct: It carries hile from the gall bladder and empties it into the tipper part of the small intestine. Common hepatic duct: It drains bile from the liver. It helps in transportation of waste from liver and helps in digestion by releasing bile.
[Note: Labels (A) and (O) have been modified for the better understanding of the students]
![]()
Practical / Project : Here are the events in the process of digestion. Fill in the blanks and complete the flow chart.
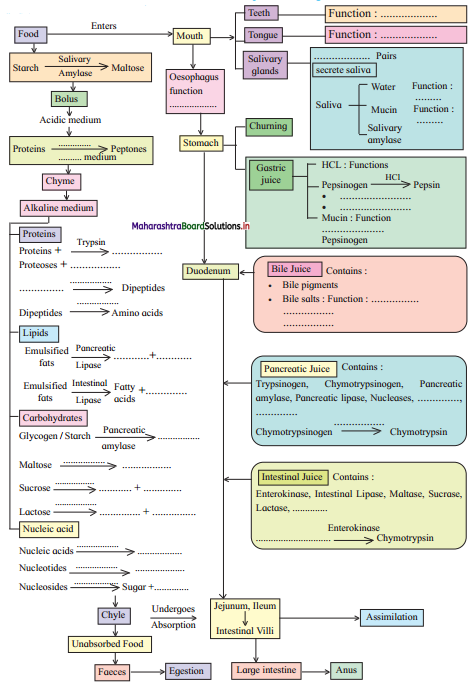
Answer:
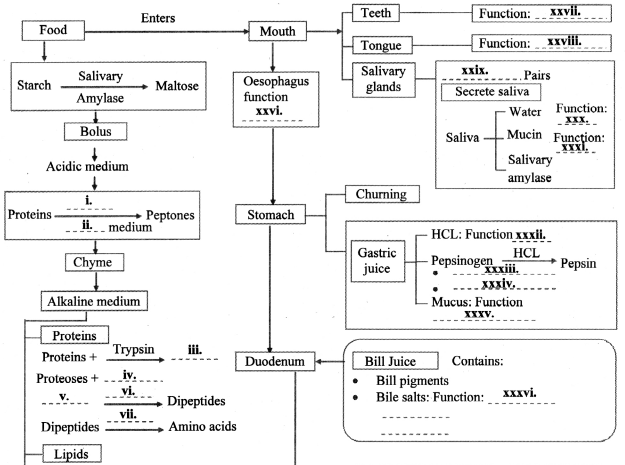
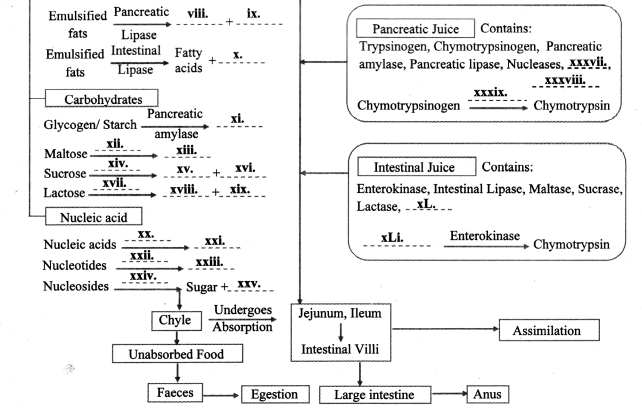
11th Biology Digest Chapter 14 Human Nutrition Intext Questions and Answers
Can you recall? (Textbook Page No. 161)
Question 1.
What is nutrition?
Answer:
- Nutrition is the sum of the processes by which an organism consumes and utilizes food substances,
- WHO defines nutrition as the intake of food, considered in relation to the body’s dietary needs.
- The term nutrition includes the process like ingestion, digestion, absorption, assimilation and egestion.
Question 2.
Enlist life processes that provide us energy to perform different activities.
Answer:
The life processes which are essential and provide us energy are nutrition and respiration.
Think about it (Textbook Page No. 161)
Question 1.
Our diet includes all necessary nutrients. Still we need to digest it. Why is it so?
Answer:
- Digestion is a very important process of converting complex, noil-diffusible and non-absorbable food substances into simple, diffusible and assimilable substances.
- Our diet includes all necessary nutrients, which are in the form of complex substances like carbohydrates, proteins, fats and vitamins.
- These complex substances are converted into simple, diffusible and assimilable substances through the process of digestion.
Hence, there is a need for digestion of food.
![]()
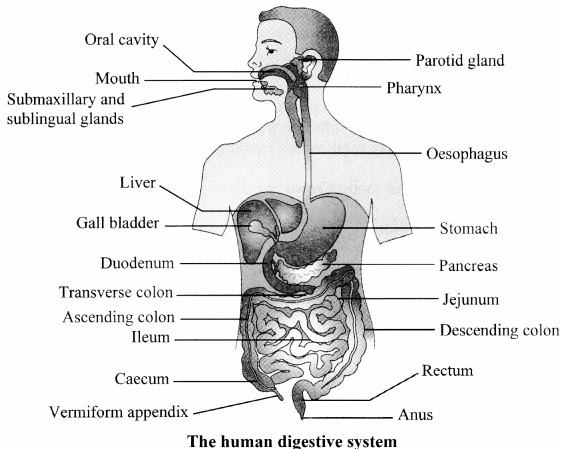
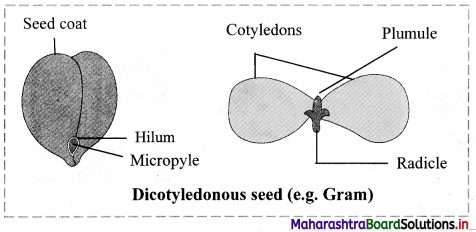
Human Digestive System (Textbook Page No. 161)
Question 1.
Label the diagram
Answer:
Do you know? (Textbook Page No. 162)
Question 1.
Who controls the deglutition?
Answer:
The process of swallowing is called deglutition. Medulla oblongata controls the deglutition.
Question 2.
Is deglutition voluntary or involuntary?
Answer:
- Deglutition consists of three phases: oral phase, pharyngeal phase and oesophagal phase.
- The oral phase is voluntary whereas the pharyngeal and oesophagal phases are involuntary.
[Source: Goya!, R. K., & Mashimo, H. (2006,.). Physio!o’ of oral, pharyngeal, and esophageal motility. GI Motility online.]
Use your brain power (Textbook Page No. 165)
Question 1.
Draw a neat labelled diagram of human alimentary canal and associated glands in situ.
Answer:

Question 2.
Write a note on human dentition.
Answer:
- Human dentition is described as thecodont, diphyodont and heterodont.
- It is called thecodont type because each tooth is fixed in a separate socket present in the jaw bones by gomphosis type of joint.
- It is called diphyodont type because we get only two sets of teeth, milk teeth and permanent teeth.
- It is called heterodont type because humans have four different type of teeth like incisors, canines, premolars and molars.
![]()
Question 3.
Muscularis layer in stomach is thicker than that in intestine. Why is it so?
Answer:
Muscularis layer in stomach is thicker than that of intestine because food is churned and gastric juices are mixed in the stomach whereas in intestine only absorption takes place.
Question 4.
Liver is a vital organ. Justify.
Answer:
- Kupffer cells of liver destroy toxic substances, dead and worn-out blood cells and microorganisms.
- Bile juice secreted by liver emulsifies fats and makes food alkaline.’
- Liver stores excess of glucose in the form of glycogen.
- Deamination of excess amino acids to ammonia and its further conversion to urea takes place in liver.
- Synthesis of vitamins A, D, K and BI2 takes place in liver.
- It also produces blood proteins like prothrombin and fibrinogen.
- During early development, it acts as haemopoietic organ.
Therefore, liver is a vital organ.
Internet my friend: (Textbook Page No. 171)
Question 1.
Collect the different videos of functioning of digestive system,
Answer:
[Note: Students can scan the adjacent Q.R code to get conceptual clarity with the aid of a relevant video.]

Find out (Textbook Page No. 162)
Question 1.
What will be the dental formula of a three years old child?
Answer:
The dental formula of a three-year-old child will be: I \(\frac{2}{2}\), C \(\frac{1}{1}\), M \(\frac{2}{2}\) = \(\frac{2,1,2}{2,1,2}\)
i. e. 5 × 2 = 10 teeth in each jaw = 20 teeth.
As a child has 20 teeth by the age of three.
![]()
Question 2.
What is dental caries and dental plaque? How can one avoid it?
Answer:
- Dental caries are tooth decay or cavities caused by acids secreted by bacteria. Dental caries may be yellow or black in color.
- Dental plaques also known as tooth plaque is a soft, sticky film which forms on the teeth regularly. It is colourless to pale yellow in colour.
- Tooth decay and dental plaque can be prevented by brushing teeth twice a day with a fluoride containing tooth paste.
- Rinsing mouth thoroughly with a mouth wash and use of dental floss or interdental cleaners to clean teeth daily can help to avoid dental caries and dental plaque.
Internet my friend (Textbook Page No. 162)
Question 1.
Find out the role of orthodontist and dental technician.
Answer:
a. Orthodontics is a specialization in dental profession. Orthodontist straightens the crooked teeth, locates problem in patients’ teeth and their overall oral development. They might use X-rays, plaster molds or dental appliances like retainers and space maintainers to correct the problems,
b. Dental technicians are the ones which improves patients’ appearance, ability to chew and speech. They make dentures, crowns, bridges and dental braces.
Question 2.
What is a root canal treatment?
Answer:
- Root canal treatment is also known as endodontic treatment.
- It is a dental treatment of removing infection from inside of a tooth.
- Root canal is hollow section of tooth which contains the nerve tissue, blood vessels and other cells, this is also known as pulps.
- Crown and root are a part of tooth. Crown is present above the gum while root is embedded in the gum.
e. Pulp which is present inside the root canal nourishes the tooth and provides moisture to the surrounding material. - The nerves present inside the pulp sense hot cold temperatures as pain.
- First step of a root canal treatment is removal of dead pulp tissues by making a hole on the surface of tooth.
- In second step, the dentist cleans and decontaminates the area and fills the hollow area with adhesive cement in order to seal the canal completely.
- The tooth is dead after the therapy and the patient no longer feel any pain but the tooth becomes more fragile than ever.
- The last step of root canal is adding a crown or filling. Until the crown or filling is complete, patient is not supposed to chew or bite using that tooth. After the crown or filling patient can use that tooth as before.
Find out (Textbook Page No. 163)
Question 1.
You must have heard about appendicitis. It is inflammation of appendix. Find more information about this disorder.
Answer:
- Appendicitis is a condition where there is inflammation of appendix.
- Appendix is a vestigial organ. It is a linger shaped pouch that projects from colon on the lower right side of the abdomen.
- Appendicitis pain is very severe. It initially starts from the navel and then moves.
- It occurs in the people of age group between 10 to 30.
- Surgical removal is the standard treatment for appendicitis.
- Symptoms: Nausea and vomiting, loss of appetite, low grade fever, constipation, abdominal bloating, severe pain in the right side of the abdomen.
- Appendicitis is caused when there is blockage in the lining of the appendix that results in infection. The bacteria multiply rapidly and causes inflammation and it is then filled with pus.
- If not treated properly appendix can rupture which can lead to further complications.
[Students can use above answer for reference and find more information about appendicitis.]
![]()
Question 2.
What is heartburn? Why do we take antacids to control it?
Answer:
Heart burn is a problem created when stomach contents (acid) are forced back up to oesophagus. It causes a burning pain in lower chest.
Antacids are bases and help to treat heartburn by neutralizing the stomach acid. The key ingredients of antacids are calcium carbonate, magnesium hydroxide, aluminium hydroxide or sodium bicarbonate.
Activity (Textbook Page No. 163)
Make a model of human digestive system in a group.
Answer:
[Students are expected to perform this activity on their own.]
Always Remember (Textbook Page No. 166)
Question 1.
Food remains for a very short time in mouth but action of salivary amylase continues for further IS to 30 minutes till gastric juice mixes with food in the stomach. Why do you think it stops after the food gets mixed with gastric juice?
Answer:
- The gastric juices are mixed with food in the stomach.
- The pH of the stomach is 1.0-2.0 which is very acidic. Such high level of acidity leads to denaturation of salivary amylase’s protein structure.
- On the other hand, pH 6.8 is required for salivary amylase to carry out the activity which is not found in stomach. Thus, activity of salivary amylase is stopped when food is mixed with gastric juice.
Internet my friend (Textbook Page No. 167)
Question 1.
How are bile pigments formed?
Answer:
- When old and worn out red blood cells are destroyed by macrophages in liver, the globin portion of hemoglobin is split off and heme is converted to biliverdin.
- Most of this biliverdin is converted to bilirubin, which gives bile its major pigmentation.
[Source http://www.biologydiscussion.com/human-physiology/digestive-system/bile-pigments/bile-pigments-origin-and-formation-digestive-juice-human-biology/81803]
![]()
Think about it (Textbook Page No. 167)
Question 1.
How can I keep my pancreas healthy? Can a person live without pancreas?
Answer:
- Pancreas can be kept healthy by:
- Eating proper balanced and low-fat diet, with plenty of whole grains, fruits and vegetables.
- Regular exercise and maintaining a healthy weight.
- Limiting alcohol consumption and avoid smoking.
- Adequate intake of water.
- Regular checkups.
- The pancreas is a gland that secretes digestive enzymes and insulin which is needed for a person to survive.
- Without pancreas the person will develop diabetes and will have to take insulin for the rest of the life.
- Without pancreas the body’s ability to absorb nutrients also decreases.
Hence, though a person can survive without pancreas he may have to remain dependent on the medicines for survival.
Do it yourself? (Textbook Page No. 167)
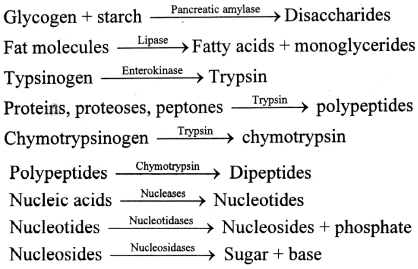
Question 1.
You have studied the representation of enzymatic actions in the form of reactions.
Write the reactions of pancreatic enzymes.
Answer:
Do it yourself (Textbook Page No. 168)
Question 1.
Observe the following reactions and explain in words.
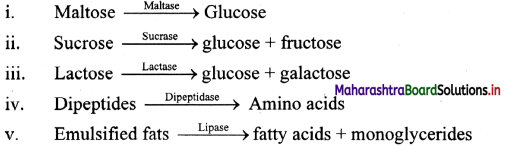
Answer:
- Maltase acts on maltose to form glucose.
- Sucrase acts on sucrose to form glucose and fructose.
- Lactase acts on lactose to form glucose and galactose.
- Dipeptidase acts on dipeptides to form amino acids.
- Emulsified fats are converted into fatty acids and glycerol by lipase.
Use your brain power (Textbook Page No. 168)
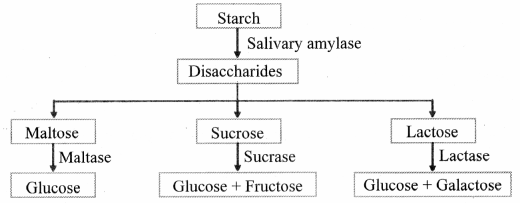
Question 1.
Make a flow chart for digestion of carbohydrate.
Answer:
![]()
Question 2.
What is a proenzyme? Enlist various proenzymes involved in process of digestion and state their function.
Answer:
Proenzymes are synthesized in cells as an inactive precursor that undergo some modification before becoming catalytically active.
The various proenzymes involved in process of digestion are as follows:
- Pepsinogen: Pepsinogen when converted into its active form pepsin acts on proteins to form peptones and proteoses.
- Trypsinogen: Trypsinogen when converted to it active form trypsin converts proteins, proteoses and peptones to polypeptides.
- Chymotrypsinogen: Chymotrypsinogen when converted to active form chymotrypsin it converts polypeptides to dipeptides.
Question 3.
Differentiate between Chyme and Chyle.
Answer:
| No. | Chyme | Chyle |
| a. | Chyme is a semi-fluid acidic mass of partially digested food. | Chyle is an alkaline slurry which contains various nutrients ready for absorption. |
| b. | Chyme leaves stomach and enters the small intestine. | Chyle leaves small intestine and enters large intestine. |
Question 4.
Digestion of fats take place only after the food reaches small intestine. Give reason.
Answer:
Digestion of fats takes place in small intestine because the presence of fats in small intestine stimulates the release of pancreatic lipase from pancreas and bile from liver. Pancreatic lipases hydrolyze fat molecules into fatty acids and monoglycerides and bile brings about emulsification of fats. Therefore, digestion of fats occur when food reaches small intestine.
Observe and Discuss (Textbook Page No. 169)
Question 1.
Action of digestive juice in your group.
Answer:
|
Digestive juices |
Action |
| Saliva | Saliva contains salivary amylase which breaks down starch into maltose. |
| Gastric juice | HC1 breaks converts inactive pepsinogen into its active form pepsin. Pepsin then breakdown proteins into peptones and proteoses. |
| Pancreatic juice | Pancreatic amylase acts on glycogen and starch and converts those into disaccharides. Enterokinase converts trypsinogen into trypsin (active form). Trypsin converts proteins, proteoses, peptones to polypeptides. Chymotrypsin converts polypeptides to dipeptides. Nucleases digest nucleic acids to pentose sugar. |
| Intestinal enzymes | Maltase converts maltose to glucose. Sucrase converts sucrose to glucose and fructose. Lactase converts lactose to glucose and galactose. Dipeptidases converts dipeptides to amino acids. Lipase converts emulsified fats into fatty acids and monoglycerides. |
| Bile juice | It brings about emulsification of fats. |
Can you recall? (Textbook Page no. 170)
Question 1.
What is balanced diet?
Answer:
Balanced diet is a diet which contains proper amount of carbohydrates, fats, vitamins, proteins and minerals to maintain a good health.
![]()
Question 2.
Explain the terms undernourished, over-nourished and malnourished in details.
Answer:
- Undernourished: When supply of nutrients is less than the minimum amount of nutrients or food required for good health is called undernourished.
- Over-nourished: The intake of nutrients is excessive. In over-nourished the amount of nutrients exceeds the amount required for normal growth.
- Malnourished: Malnourished is a condition where a person’s diet does not contain right amount of nutrients.
Do you know? (Textbook Page No. 170)
Question 1.
What is gross calorific value?
Answer:
The amount of heat liberated by complete combustion of lg food in a bomb calorimeter is termed as gross calorific (gross energy) value.
Question 2.
What is physiological value?
Answer:
The actual energy produced by 1 g food is its physiological value.
Question 3.
Name the following
Energy content of food in animals is expressed in terms of?
Answer:
Heat Energy
Question 4.
Complete the following table representing Gross calorific value and physiological value of food component.
|
Food Component |
Gross calorific value (Kcal/g) |
Physiological value (Kcal/g) |
| Fats | (A) | 9.0 |
| (B) | 5.65 | 4.0 |
| Carbohydrates | (C) | (D) |
Answer:
|
Food Component |
Gross calorific value (Kcal/g) |
Physiological value (Kcal/g) |
| Fats | 9.45 | 9.0 |
| Proteins | 5.65 | 4.0 |
| Carbohydrates | 4.1 | 4.0 |
Find out (Textbook Page No. 171)
Question 1.
Find out the status of nialnutrition among children in Maharashtra and efforts taken by the government to overcome the situation. Search for various NGOs working in this field.
Answer:
93,783 children have been diagnosed with severe acute malnutrition and 5.7 lakh with moderate acute malnutrition in Maharashtra.
Steps taken by government to overcome malnutrition:
- Promotion of infant and young child feeding practices.
- Management of malnutrition at community and facility level by trained service providers.
- Treatment of children with severe acute malnutrition at special units called the Nutrition Rehabilitation Centres (NRCs), set up at public health facilities.
- A special program to combat micronutrient deficiencies of Vitamin A, Iron and Folic acid.
- The initiatives like Mother and Child protection card, village health and nutrition days, are taken by the government for addressing the nutrition concerns in children, pregnant women and lactating mothers.
Various NCOs working in this field:
- Akshay Patra
- Fight Hunger Foundation,
- Feeding India,
- No Hungry child
[Source: http://pib.nic.in/newsite/PrintRelease.aspx?relid=l 13725; https://yourstory.com/2016/10/world- food-day-ngosj
[Note: Students can use above answer as reference and find more information from the internet.]
![]()
Question 2.
Are jaundice and hepatitis same disorders?
Answer:
Jaundice and Hepatitis are two different disorders.
Jaundice: Jaundice occurs when the rate of bilirubin production exceeds the rate of its elimination. It causes yellowing of skin and eyes.
Hepatitis: It is a disease where there is inflammation of liver. It may be caused because of infection, over alcohol consumption, immune system disorder etc.
Do you know (Textbook Page No. 171)
Question 1.
Alcoholism causes different disorders of liver like steatosis (fatty liver), alcoholic hepatitis, fibrosis and cirrhosis. Collect more information on these disorders and try to increase awareness against alcoholism in society. Collect information about NGOs working against alcoholism.
Answer:
Steatosis (fatty liver): Steatosis is accumulation of fat in the liver. Treatment can help but it cannot be cured. Major risk factors are obesity and Diabetes type II, it is also associated with excessive alcohol consumption. Fatigue, weight loss and abdominal pain are some symptoms. It is a benign condition but in very smaller number of patients it can lead to liver failure. Treatment involves diet and exercise to reduce obesity.
Alcoholic hepatitis: Alcoholic Hepatitis is liver inflammation caused by excessive consumption of alcohol. It occurs in people who drink heavily for many years. Symptoms like yellowing of skin and eye, accumulation of fluid in stomach which leads to increase in stomach size. Treatments like completely stopping of alcohol consumption, hydration and nutrition care are carried out. Administration of steroid drugs reduces liver inflammation.
Fibrosis: There is significant scarring of liver tissue in this condition. Fibrosis itself does not cause any symptoms. Diagnosis includes doctor’s evaluation, blood tests and imaging tests, liver biopsy. Treatments include stopping the consumption of alcohol. There are no such effective drugs for curing of fibrosis.
Cirrhosis: It is a chronic liver damage caused due to various reasons which leads to irreversible scarring of liver and liver failure. Causes of cirrhosis are chronic alcohol abuse and hepatitis. Patients may experience fatigue, weakness and weight loss. In later stages, patients may develop jaundice, abdominal swelling and gastrointestinal bleeding. In advanced stage, a liver transplant is required.
NGOs working against alcoholism:
- Muktangan Rehabilitation Centre
- Anmol Jeevan Foundation
- Sankalp Rehabilitation Trust
- Kripa Foundation
- Harmony Foundation
- Hands for you Rehab Centre
11th Std Biology Questions And Answers:
- Morphology of Flowering Plants Class 9 Biology Questions And Answers
- Animal Tissue Class 9 Biology Questions And Answers
- Study of Animal Type : Cockroach Class 9 Biology Questions And Answers
- Photosynthesis Class 9 Biology Questions And Answers
- Respiration and Energy Transfer Class 9 Biology Questions And Answers
- Human Nutrition Class 9 Biology Questions And Answers
- Excretion and Osmoregulation Class 9 Biology Questions And Answers
- CSkeleton and Movement Class 9 Biology Questions And Answers





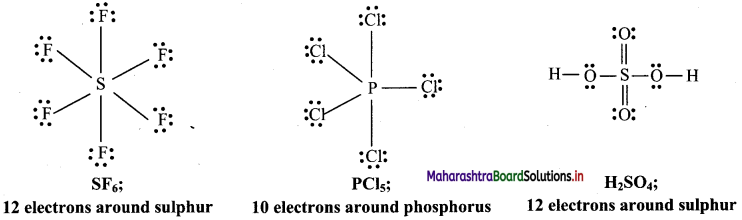
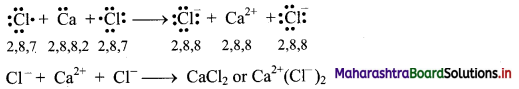
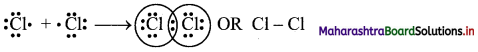
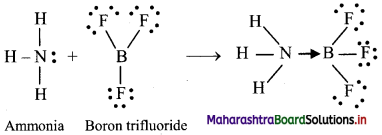
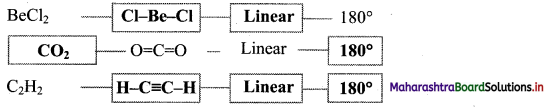
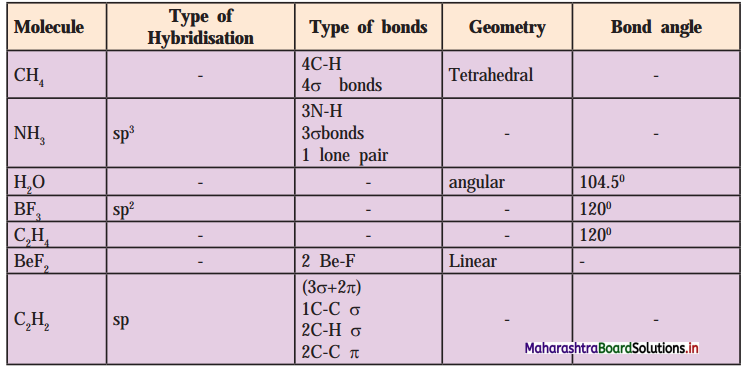
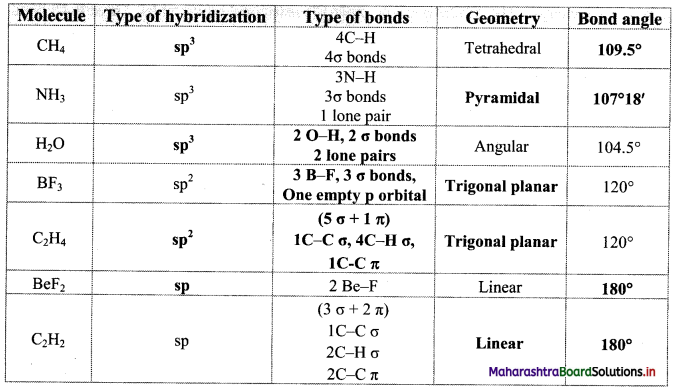
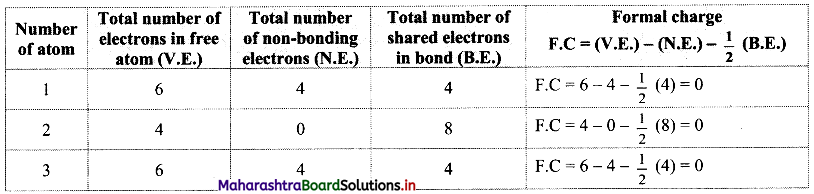


 Answer:
Answer: