Balbharti Maharashtra State Board 12th Biology Important Questions Chapter 2 Reproduction in Lower and Higher Animals Important Questions and Answers.
Maharashtra State Board 12th Biology Important Questions Chapter 2 Reproduction in Lower and Higher Animals
Multiple-choice questions
Question 1.
Gemmule formation takes place in ……………….
(a) Hydra
(b) Spongilla
(c) Planaria
(d) Human being
Answer:
(b) Spongilla
Question 2.
Which part of ovary in mammals acts as an endocrine gland after ovulation?
(a) stroma
(b) germinal epithelium
(c) vitelline membrane
(d) graafian follicle
Answer:
(d) graafian follicle

Question 3.
Cessation of menstrual cycle in female is called ……………….
(a) lactation
(b) ovulation
(c) menarche
(d) menopause
Answer:
(d) menopause
Question 4.
Capacitation of sperms occurs in ……………….
(a) vas deferens
(b) vas efferens
(c) vagina
(d) ejaculatory duct
Answer:
(c) vagina
Question 5.
How many sperms are formed from a secondary spermatocyte ?
(a) 4
(b) 8
(c) 2
(d) 1
Answer:
(c) 2
Question 6.
The middle piece of the sperm contains ……………….
(a) proximal centriole
(b) nucleus
(c) mitochondria
(d) distal centriole
Answer:
(c) mitochondria
Question 7.
About which day in a normal human menstrual cycle does rapid secretion of LH (popularly called LH surge) normally occurs ?
(a) 14th day
(b) 20th day
(c) 5th day
(d) 11th day
Answer:
(a) 14th day
Question 8.
Morphogenetic movements occur during ……………….
(a) blastulation
(b) gastrulation
(c) fertilization
(d) cleavage
Answer:
(b) gastrulation
Question 9.
The technique used to block the passage of sperm in male is ……………….
(a) tubectomy
(b) vasectomy
(c) coitus interruptus
(d) rhythm method
Answer:
(b) vasectomy
Question 10.
Planaria reproduces asexually through ……………….
(a) budding
(b) gemmule formation
(c) regeneration
(d) binary fission
Answer:
(c) regeneration
Question 11.
The role of Leydig cells is ……………….
(a) nourishment of sperms
(b) to give motility to sperms
(c) synthesis of testosterone
(d) to undergo spermatogenesis
Answer:
(c) synthesis of testosterone
Question 12.
Chancre are the primary lesions caused by ……………….
(a) Neisseria gonorrhoeae
(b) Treponema pallidum
(c) Plasmodium vivax
(d) Salmonella typhi
Answer:
(b) Treponema pallidum
Question 13.
Smooth muscles lining the wall of scrotum are called ……………….
(a) detrusor muscles
(b) dartos muscles
(c) gluteal muscles
(d) latissimus dorsi muscles
Answer:
(b) dartos muscles
Question 14.
The trophoblast cells in contact with embryonal knob are called ……………….
(a) inner mass cells
(b) blastomere
(c) amniogenic cells
(d) cells of Rauber
Answer:
(d) cells of Rauber
Question 15.
The external layer of collagenous connective tissue of human testis is ……………….
(a) tunica vasculosa
(b) tunica vaginalis
(c) tunica granulosa
(d) tunica albuginea
Answer:
(d) tunica albuginea
Question 16.
Which of the following is mesodermal in origin ?
(a) Retina
(b) Enamel of teeth
(c) Heart
(d) Liver
Answer:
(c) Heart
Question 17.
Pregnancy in second trimester is maintained by ……………….
(a) LH (luteinizing hormone)
(b) progesterone
(c) estrogen
(d) hCG (human Chorionic Gonadotropin)
Answer:
(b) progesterone
Question 18.
In human foetus, the heart begins to beat at developmental age of ……………….
(a) 4th week
(b) 3rd week
(c) 6th week
(d) 8th week
Answer:
(c) 6th week
Question 19.
……………… contribute about 60% of the total volume of the semen.
(a) Prostate gland
(b) Cowper’s glands
(c) Seminal vesicles
(d) Bartholin’s glands
Answer:
(c) Seminal vesicles
Question 20.
Which of the following is hormone releasing IUD?
(a) Lippes loop
(b) Cu 7
(c) LNG 20
(d) Multiload 375
Answer:
(c) LNG 20
Question 21.
Which of the following is incorrect regarding vasectomy?
(a) Vasa deferentia is cut and tied
(b) Irreversible sterility
(c) No sperm occurs in seminal fluid
(d) No sperm occurs in epididymis
Answer:
(d) No sperm occurs in epididymis
Question 22.
The test-tube baby programme employs which one of the following techniques?
(a) Gamete intra fallopian transfer (GIFT)
(b) Zygote intra fallopian transfer (ZIFT)
(c) Intra cytoplasmic sperm injection (ICSI)
(d) Intra uterine insemination (IUI)
Answer:
(b) Zygote intra fallopian transfer (ZIFT)
Question 23.
Medical Termination of Pregnancy (MTP) is considered safe up to how many weeks of pregnancy?
(a) 8 weeks
(b) 12 weeks
(c) 24 weeks
(d) 6 weeks
Answer:
(b) 12 weeks
Question 24.
‘Saheli? an oral contraceptive pill is to be taken ……………….
(a) daily
(b) weekly
(c) quarterly
(d) monthly
Answer:
(b) weekly
Question 25.
The role of copper releasing IUDs is to ……………….
(a) inhibit ovulation
(b) prevent fertilization
(c) inhibit implantation of blastocyst
(d) inhibit gametogenesis
Answer:
(b) prevent fertilization
Question 26.
The phenomenon of nuclear fusion of sperm and egg is known as ……………….
(a) karyogamy
(b) parthenogenesis
(c) vitellogenesis
(d) oogenesis
Answer:
(a) karyogamy
Question 27.
Acrosome of spermatozoa is formed from ……………….
(a) lysosomes
(b) Golgi bodies
(c) ribosomes
(d) mitochondria
Answer:
(b) Golgi bodies
Question 28.
Which of the following undergoes spermiogenesis ?
(a) Spermatids
(b) Spermatogonia
(c) Primary spermatocytes
(d) Secondary spermatocytes
Answer:
(a) Spermatids
Question 29.
In mammals, the estrogens are secreted by the graafian follicle from its ……………….
(a) theca externa
(b) theca interna
(c) membrane granulosa
(d) corona radiata
Answer:
(b) theca interna
Question 30.
Which hormone is essential for maintenance of the endometrium of uterus?
(a) FSH
(b) LH
(c) Progesterone
(d) Estrogen
Answer:
(c) Progesterone
Question 31.
Which of the following cells during gametogenesis is normally diploid?
(a) Spermatid
(b) Spermatogonia
(c) Second polar body
(d) Secondary oocyte
Answer:
(b) Spermatogonia
Question 32.
Fertilization takes place at ……………….
(a) cervix
(b) ampulla
(c) isthmus
(d) vagina
Answer:
(b) ampulla
Question 33.
In mammals, failure of testes to descend into scrotum is known as ……………….
(a) paedogenesis
(b) castration
(c) cryptorchidism
(d) impotency
Answer:
(c) cryptorchidism

Question 34.
Polar body is produced during the formation of ……………….
(a) sperm
(b) secondary oocyte
(c) oogonium
(d) spermatocytes
Answer:
(b) secondary oocyte
Question 35.
Menstrual flow occurs due to lack of ……………….
(a) vasopressin
(b) progesterone
(c) FSH
(d) oxytocin
Answer:
(b) progesterone
Question 36.
Approximately how many eggs are produced by a normal healthy human female up to the age of 25 years if the age of menarche is 12 years?
(a) 169
(b) 416
(c) 240
(d) 100
Answer:
(a) 169
Question 37.
In humans, at the end of the first meiotic division, the male germ cells differentiate into the ……………….
(a) spermatids
(b) spermatozoa
(c) primary spermatocytes
(d) secondary spermatocytes
Answer:
(d) Secondary spermotocytes
Question 38.
The part that carries sperms from testis to epididymis is ……………….
(a) rete testis
(b) vasa efferentia
(c) vasa differentia
(d) ejaculatory ducts
Answer:
(c) vasa differentia
Question 39.
Which period of menstrual cycle is called risky period of conception?
(a) 3rd to 7th day
(b) 7th to 13th day
(c) 10th to 17th day
(d) 15th to 25th day
Answer:
(c) 10th to 17th day
Question 40.
Which hormone confirms pregnancy?
(a) Progesterone
(b) Estrogen
(c) hCG
(d) LH
Ans
(c) hCG
Match the columns
Question 1.
| Column I [Organs] |
Column II [Functions] |
| (1) Epididymis |
(a) Transport of sperms |
| (2) Sertoli cells |
(b) Copulatory organ |
| (3) Vas deferens |
(c) Nourishment to developing sperms |
| (4) Penis |
(d) Maturation of sperms |
Answer:
| Column I [Organs] |
Column II [Functions] |
| (1) Epididymis |
(d) Maturation of sperms |
| (2) Sertoli cells |
(c) Nourishment to developing sperms |
| (3) Vas deferens |
(a) Transport of sperms |
| (4) Penis |
(b) Copulatory organ |
Question 2.
| Column I [Organ/cells] |
Column II [Hormones] |
| (1) Corpus luteum |
(a) hCG |
| (2) Interstitial cells / Leydig’s cells |
(b) Estrogen |
| (3) Placenta |
(c) Progesterone |
| (4) Graafian follicle |
(d) Testosterone |
Answer:
| Column I [Organ/cells] |
Column II [Hormones] |
| (1) Corpus luteum |
(c) Progesterone |
| (2) Interstitial cells / Leydig’s cells |
(d) Testosterone |
| (3) Placenta |
(a) hCG |
| (4) Graafian follicle |
(b) Estrogen |
Question 3.
| Column I |
Column II |
| (1) Graafian follicle |
(a) Site of implantation |
| (2) Uterus |
(b) Birth canal |
| (3) Fallopian tube |
(c) Site of fertilization |
| (4) Vagina |
(d) Release of secondary oocyte |
Answer:
| Column I |
Column II |
| (1) Graafian follicle |
(d) Release of secondary oocyte |
| (2) Uterus |
(a) Site of implantation |
| (3) Fallopian tube |
(c) Site of fertilization |
| (4) Vagina |
(b) Birth canal |
Question 4.
| Column I [Phases] |
Column II [Hormonal changes] |
| (1) Menstrual phase |
(a) Rapid secretion of LH |
| (2) Proliferative phase |
(b) Increased level of FSH and estrogen |
| (3) Ovulatory phase |
(c) Increased level of progesterone |
| (4) Secretory phase |
(d) Decrease in progesterone and estrogen |
Answer:
| Column I [Phases] |
Column II [Hormonal changes] |
| (1) Menstrual phase |
(d) Decrease in progesterone and estrogen |
| (2) Proliferative phase |
(b) Increased level of FSH and estrogen |
| (3) Ovulatory phase |
(a) Rapid secretion of LH |
| (4) Secretory phase |
(c) Increased level of progesterone |
Question 5.
| Column I |
Column II |
| (1) Acrosome |
(a) Completion of IInd meiotic division of secondary oocyte |
| (2) Penetration of sperm into ovum |
(b) Dissolution of zona pellucida |
| (3) Formation of fertilization membrane |
(c) Secretion of Hyaluronidase |
| (4) Acrosin / Zona lysine |
(d) Prevention of polyspermy |
Answer:
| Column I |
Column II |
| (1) Acrosome |
(c) Secretion of Hyaluronidase |
| (2) Penetration of sperm into ovum |
(a) Completion of IInd meiotic division of secondary oocyte |
| (3) Formation of fertilization membrane |
(d) Prevention of polyspermy |
| (4) Acrosin / Zona lysine |
(b) Dissolution of zona pellucida |
Question 6.
| Column I |
Column II |
| (1) Parturition |
(a) Attachment of embryo to endometrium |
| (2) Gestation |
(b) Release of egg from Graafian follicle |
| (3) Ovulation |
(c) Delivery of baby from uterus |
| (4) Implantation |
(d) Duration between pregnancy and birth |
Answer:
| Column I |
Column II |
| (1) Parturition |
(c) Delivery of baby from uterus |
| (2) Gestation |
(d) Duration between pregnancy and birth |
| (3) Ovulation |
(b) Release of egg from Graafian follicle |
| (4) Implantation |
(a) Attachment of embryo to endometrium |
Question 7.
| Column I [Contraceptive method] |
Column II [Mode of action] |
| (1) Pill |
(a) Prevents sperms reaching cervix |
| (2) Condom |
(b) Prevents implantation |
| (3) Vasectomy |
(c) Prevents ovulation |
| (4) Copper T |
(d) Semen contains no sperms |
Answer:
| Column I [Contraceptive method] |
Column II [Mode of action] |
| (1) Pill |
(c) Prevents ovulation |
| (2) Condom |
(a) Prevents sperms reaching cervix |
| (3) Vasectomy |
(d) Semen contains no sperms |
| (4) Copper T |
(b) Prevents implantation |
Question 8.
| Column I |
Column II |
| (1) Mechanical means |
(a) Saheli |
| (2) Physiological device |
(b) Jellies |
| (3) Chemical device |
(c) Vasectomy |
| (4) Permanent method |
(d) Diaphragm |
Answer:
| Column I |
Column II |
| (1) Mechanical means |
(d) Diaphragm |
| (2) Physiological device |
(a) Saheli |
| (3) Chemical device |
(b) Jellies |
| (4) Permanent method |
(c) Vasectomy |
Classify the following to form Column B as per the category given in Column A.
Question 1.
Classify the following contraceptives given below as per Column ‘A’ and complete Column ‘B’. Select from the given options:
(i) Foams
(ii) Lippe’s loop
(iii) Cervical caps
(iv) Multiload 375
(v) Diaphragms
(vi) Jellie
| Column A |
Column B |
| (1) Mechanical means |
————–, ———— |
| (2) Chemical means |
————-, ————- |
| (3) Intra-uterine device |
————-, ———— |
Answer:
| Column A |
Column B |
| (1) Mechanical means |
Cervical caps, Diaphragms |
| (2) Chemical means |
Foams, Jellies |
| (3) Intra-uterine device |
Lippe’s loop, Multiload 375 |
Question 2.
Classify the following components of semen given below as per Column ‘A’ and complete the Column ‘B’. Select from the given options
(i) Acid phosphatase
(ii) Mucous like fluid
(iii) Prostaglandins
(iv) Citric acid
(v) Fructose
(vi) Fibrinogen
| Column A |
Column B |
| (1) Seminal fluid |
————–, ———— |
| (2) Prostatic fluid |
————-, ————- |
| (3) Fluid from Cowper’s gland |
————-, ———— |
Answer:
| Column A |
Column B |
| (1) Seminal fluid |
Prostaglandins, Fructose, Fibrinogen |
| (2) Prostatic fluid |
Acid phosphatase, Citric acid |
| (3) Fluid from Cowper’s gland |
Mucous like fluid |
Very short answer questions
Question 1.
How many sperms are present in single ejaculation?
Answer:
A single ejaculation contains about 400 millions of sperms.
Question 2.
What is gemmule? How is gemmule formed ?
Answer:
Gemmule is an internal bud formed by aggregation of archeocytes in sponges to overcome unfavourable season.
Question 3.
What is cryptorchidism?
Answer:
Failure of testis to descend into scrotum leading to sterility is called cryptorchidism.

Question 4.
What is the beginning of the menstrual cycle and cessation of menstrual cycle respectively called?
Answer:
The beginning of the menstrual cycle is called menarche while cessation of menstrual cycle is called menopause.
Question 5.
Which men have an increased risk of prostate cancer?
Answer:
Men who are over 50 years of age and have a daily high consumption of fat have an increased risk of prostate cancer.
Question 6.
What is capacitation with reference to sperm?
Answer:
Changes in a mammalian sperm which prepare it for fertilization of ovum is called capacitation.
Question 7.
Give any two examples each of seasonal breeders and continuous breeders among sexually reproducing animals.
Answer:
Example of seasonal breeders : Goat, Sheep and Donkey.
Example of continuous breeders : Humans, apes.
Question 8.
What does IUCD indicate?
Answer:
IUCD means Intra Uterine Contraceptive Device.
Question 9.
What is full form of IVF?
Answer:
IVF means In Vitro Fertilization.
Question 10.
From which germinal layers the nervous system is derived?
Answer:
The nervous system is derived from ectoderm.
Question 11.
A mother of a one-year-old child wanted to space her second child. Her doctor suggested ‘Copper-T’. Explain its contraceptive action.
Answer:
Copper ions released from ‘Copper-T’ suppress sperm motility and the fertilizing capacity of sperms.
Question 12.
Which options are available for infertile couples to have child?
Answer:
Infertile couples have many options to have a child such as fertility drugs, modern techniques such as IVE ZIFT, GIFT, ICSI, artificial insemination, IUI, using surrogate mother or taking the sperm from sperm bank.
Question 13.
How many primary spermatocytes and oocytes are required for the formation of 100 spermatozoa and ova?
Answer:
25 Primary spermatocytes and 100 primary oocytes will be required for the formation of 100 spermatozoa and ova respectively.
Question 14.
The entrance of fallopian tube of a lady is blocked. She wants motherhood. Which method will help her?
Answer:
The method of GIFT or Gamete Intra Fallopian Transfer is the method that will help the lady to have a child.
Question 15.
What is the role of birth control pills?
Answer:
Birth control pills are contraceptive pills that check the ovulation by inhibiting the secretion of FSH and LH.
Question 16.
In T.S. of ovary, can all stages of follicles be seen simultaneously?
Answer:
In T.S. of ovary, all the stages of follicles cannot be seen simultaneously. The stage of follicles develop alternately in the ovary as per timing of menstrual cycle under the influence of hormones of pituitary and ovaries.
Question 17.
What will be marriageable age for boy and girl as per the Indian law?
Answer:
As per the Hindu Marriage Act, minimum age for boy must be 21 years and for a girl 18 years, at the time of marriage.
Question 18.
What is MTP Act?
Answer:
MTP Act is for reducing the incidences of illegal abortions and maternal mortalities.
Question 19.
Which is the time period legally allowed by MTP ACT for terminating pregnancy?
Answer:
According to MTP Act, pregnancy may be terminated within first 12 weeks, more than 12 weeks but lesser than 20 weeks.
Give definitions of the following
Question 1.
Amphimixis
Answer:
It is the process which involves the production of offspring by the formation and fusion of gametes.
Question 2.
Gametogenesis
Answer:
The gametogenesis is the process of formation of gametes in sexually reproducing animals.
Question 3.
Spermiogenesis
Answer:
The process of transformation of non-motile and non¬functional spermatid into a functional and motile spermatozoa is called spermiogenesis.
Question 4.
Insemination
Answer:
The process of deposition of semen into the vagina of the female at the time of coitus or sexual intercourse is called insemination.
Question 5.
Cleavage
Answer:
The process of early mitotic division of the zygote to form a multicellular morula stage is called cleavage.
Question 6.
Implantation
Answer:
The process by which the blastocyst after its formation, gets implanted or embedded into the endometrium of the uterus is called implantation.
Question 7.
Gestation
Answer:
The condition of carrying one or more embryos in the uterus is called gestation.
Question 8.
Placenta
Answer:
A flattened, discoidal organ present in the uterus of pregnant mother and which acts as endocrine source and nutrition provider for growing foetus is called placenta.
Question 9.
Lactation
Answer:
The process of secretion of milk in the mammary glands and expelling it through nipples out to provide nourishment to the growing baby is called lactation.
Question 10.
Parturition
Answer:
Parturition is the process of giving birth to a baby.
Question 11.
Amniocentesis
Answer:
Amniocentesis is a process in which amniotic fluid containing foetal cells is collected using a hollow needle inserted into the uterus under ultrasound guidance.
Question 12.
Infertility
Answer:
Infertility is defined as the inability to conceive naturally after (one year of) regular unprotected intercourse.
Question 13.
IVF (In Vitro Fertilization)
Answer:
It is a process of fertilization where an egg is combined with sperm outside the body in a test tube or glass plate to form a zygote under simulated conditions in the laboratory.
Question 14.
Artificial Insemination (AI)
Answer:
It is the technique during which the sperms are collected from the male and artificially introduced into the cervix of female, for the purpose of achieving a pregnancy through in vivo fertilization (inside the body).
Question 15.
Adoption
Answer:
Adoption is a legal process by which a couple or a single parent gets legal rights, privileges and responsibilities that are associated to a biological child for the upbringing of the adopted child.
Give functions of the following
Question 1.
Corpus luteum.
Answer:
Corpus luteum is a secondary endocrine source that produces progesterone for maintaining pregnancy.
Question 2.
Scrotum.
Answer:
Scrotum protects the testis and also acts as thermoregulator.
Question 3.
Acrosome of sperm.
Answer:
Acrosome of the sperm releases hyaluronidase which digests the zona pellucida surrounding the ovum due to which sperm can fertilize the ovum.
Question 4.
Sertoli cells.
Answer:
Sertoli cells provide nourishment and surface to the sperm bundles during their development.
Question 5.
Interstitial cells / Leydig’s cells.
Answer:
Interstitial cells / Leydig’s cells secrete testosterone or androgen which is a male sex hormone.
Question 6.
Prostate gland.
Answer:
Prostate gland secretes prostatic fluid which forms 30% of semen, Citric acid and acid phosphatase present in this fluid protects the sperms from acidic environment of vagina.
Question 7.
Bulbourethral glands.
Answer:
Bulbourethral glands secrete alkaline, viscous mucus like fluid which provides lubrication during copulation.
Question 8.
Bartholin’s glands.
Answer:
Bartholin glands secrete lubricating mucus like fluid which is released in vestibule.
Question 9.
Uterus.
Answer:
- Uterus receives ovum from fallopian tubes, develops placenta and provides site for implantation of embryo.
- It provides protection and nourishment to the developing embryo.
- It also provides path for sperms to ascend.
- Due to contractions of uterus, baby is expelled out at the time of parturition.
Question 10.
Vagina
Answer:
- Vagina acts as a copulatory passage.
- It acts as a birth canal during parturition in normal delivery
- It provides the passage for menstrual flow.
Name the following
Question 1.
The canal through which the testes descend into scrotum just before birth in human male child.
Answer:
Inguinal canal
Question 2.
The structure where sperms are matured.
Answer:
Epididymis
Question 3.
The part where the sperms are produced in the testes.
Answer:
Germinal epithelium of seminiferous tubules.
Question 4.
The gland in females homologous to Cowper’s gland.
Answer:
Bartholin’s glands or Vestibular glands.
Question 5.
Type of cleavage in human zygote
Answer:
Holoblastic, radial and indeterminate
Question 6.
The developmental stage of human being which gets implanted in the endometrium of uterus.
Answer:
Blastocyst
Question 7.
Name the primates who show presence of menstrual cycle.
Answer:
Human being and Apes like gorilla, chimpanzee, orangutan, etc.
Question 8.
Structures which help in transport of secondary oocyte through uterine tube.
Answer:
Ciliated epithelium
Question 9.
Hormones produced in women only during pregnancy.
Answer:
hCG, HPL (Human Placental Lactogen) and relaxin.
Question 10.
The oral contraceptive pill which is now. a part of the National Family Programme in India.
Answer:
Saheli
Question 11.
The scientific term for the animals giving birth to live young ones.
Answer:
Viviparous

Question 12.
The site of fertilization in woman.
Answer:
Ampulla of fallopian tube
Question 13.
The trophoblast cells lying over the embryonal knob.
Answer:
Cells of Rauber
Question 14.
The muscles which form the wall of scrotum.
Answer:
- Dartos muscles
- Cremaster muscles
Question 15.
Names of erectile tissues in penis.
Answer:
- Corpora cavernosa
- Corpus spongiosum
Question 16.
Any two copper releasing IUD.
Answer:
- Copper-T, Cu 7
- Multiload 375
Question 17.
Any two hormone-releasing IUDs.
Answer:
- LNG-20
- Progestaert
Question 18.
Two methods of birth control which have high chances of failure.
Answer:
- Safe period
- Lactational amenorrhea
Question 19.
Uterine walls.
Answer:
- Perimetrium
- Myometrium
- Endometrium
Question 20.
Regions of the uterus.
Answer:
- Fundus
- Body
- Cervix
Question 21.
Parts of fallopian tubes.
Answer:
- Infundibulum
- Ampulla
- Isthmus
Question 22.
Layers of Graafian follicle which enclose antrum.
Answer:
- Theca externa
- Theca interna
- Membrana granulosa
Question 23.
Stages of cells in spermatogenesis.
Answer:
- Spermatogonia
- Primary spermatocytes
- Secondary spermatocytes
- Spermatids
- Sperms
Question 24.
Stages of cells in oogenesis.
Answer:
- Oogonia
- Primary oocytes
- Secondary oocytes
- Ootid
- Ovum
Give significance of the following
Question 1.
Fertilization.
Answer:
Significance of fertilization:
- Fertilization forms the zygote which eventually produces new offspring.
- Fertilization restores diploid number of chromosomes in the zygote as two haploid gametes come together in a zygote.
- During fertilization, centrioles are passed on to the ovum, due to this secondary oocyte can complete meiosis-II. The fertilization thus concludes the process of oogenesis.
- By fertilization the genetic characters of two parents are mixed. This leads to variation and has significance in evolution.
- Due to fertilization the sex of young one is determined.
Question 2.
Implantation.
Answer:
Gestation becomes possible due to implantation. Implantation protects the embryo and helps it to derive nourishment from the mother’s body through placenta.
Question 3.
Corpus luteum.
Answer:
- Corpus luteum is the temporary source of female hormone, progesterone.
- Corpus luteum is formed from empty Graafian follicle after the process of ovulation.
- Due to progesterone secreted from corpus luteum, the endometrial wall of uterus undergoes repair and increase in thickness.
- Progesterone is a gestational hormone and thus pregnancy is maintained if corpus luteum is well functional.
Question 4.
Fertilization membrane.
Answer:
Fertilization membrane prevents any further entry of other sperms into the egg, i.e. polyspermy is avoided.
Question 5.
Gastrulation.
Answer:
- Due to the process of gastrulation, three germinal layers, viz. ectoderm, mesoderm and endoderm are formed.
- Cells of embryonal knob become embryonic disc which develop into embryo due to gastrulation.
- Gastrulation is necessary for the formation of amniotic cavity which is filled with amniotic fluid.
Question 6.
Trophoblast of blastocyst.
Answer:
- Trophoblast cells help in absorbing nutrition for the developing embryo.
- Trophoblast cells at the embryonal knob (cells of Rauber) help in implantation of blastocyst.
- Synctiotrophoblast helps in implantation of fertilized ovum in the uterine endometrium.
Question 7.
hCG [human chorionic gonadotropin].
Answer:
hCG [human chorionic gonadotropin] is secreted in the pregnant female to extend the life of corpus luteum and stimulates its secretory activity. Presence of hCG in maternal blood and urine is an indication of pregnancy.
Question 8.
Colostrum.
Answer:
- Colostrum is the first milk which is sticky and yellowish secreted by the mammary glands soon after the parturition.
- Being high protein in its content, it nourishes the newly born child.
- The antibodies present in it helps in developing resistance for the newborn baby at a time when its own immune response is not fully developed.
Distinguish between the following
Question 1.
Asexual reproduction and Sexual reproduction.
Answer:
| Sexual reproduction |
Asexual reproduction |
| 1. Asexual reproduction requires single parent. |
1. Sexual reproduction needs two different parents. |
| 2. Meiosis does not take place in asexual reproduction. Only mitosis takes place. |
2. Sexual reproduction involves meiosis and mitosis. |
| 3. Gamete formation, fertilization and zygote formation does not take place. |
3. Gamete formation, fertilization and zygote formation are important processes in sexual reproduction. |
| 4. Progeny and parent Eire identical genetically. |
4. Progeny and parents are genetically dissimilar. |
| 5. Large number of progeny is developed by asexual reproduction. E.g. Spore formation, gemmule formation, budding, regeneration are the types of a sexual reproduction. |
5. Limited number of progeny is developed by sexual reproduction. E.g. Sexual reproduction is only by a single method. |
Question 2.
Primary sex organs and Secondary sex organs.
Answer:
| Primary sex organs |
Secondary / Accessory sex organs |
| 1. Primary sex organs produce gametes. |
1. Secondary sex organs do not produce gametes. |
| 2. Primary sex organs secrete sex hormones. |
2. Secondary sex organs do not secrete sex hormones. |
3. Development of these organs is under the control of Gonadotropins released from Pituitary.
E.g. Testes in male and Ovaries in females. |
3. Development of these organs is under the control of estrogen and progesterone in females and testosterone in males.
Eg. Prostate, seminal vesicles, vas deferens in males. Fallopian tubes, uterus and vagina in females. |
Question 3.
Vasa efferentia and Vasa deferentia.
Answer:
| Vasa efferentia |
Vasa deferentia |
| 1. Vasa efferentia arise from the rete testes and enter the epididymis. |
1. Vasa deferentia arise from the epididymis and form ejaculatory duct after the union with seminal duct. |
| 2. They are present in 15-20 number and are fine convoluted ductules. |
2. They are thick and coiled ductules present in a single pair. |
| 3. The spermatozoa are carried from rete testis to epididymis by vasa efferentia. |
3. The spermatozoa are carried from epididymis to ejaculatory ducts by vasa deferentia. |
Question 4.
Graafian follicle and Corpus luteum.
Answer:
| Graafian follicle |
Corpus luteum |
| 1. Graafian follicle is produced by the maturation of the primary follicle. |
1. Corpus luteum is produced by the cells of ruptured Graafian follicle. |
| 2. It is formed in the ovary before ovulation. |
2. It is formed in the ovary after ovulation. |
| 3. It produces the hormone estrogen. |
3. It produces the hormone progesterone. |
| 4. It has secondary oocyte surrounded by follicle cells. |
4. It has only follicle cells. |

Question 5.
Menarche and Menopause.
Answer:
| Menarche |
Menopause |
| 1. Menarche is the beginning of menstrual cycle. |
1. Menopause is the stoppage of menstrual cycle. |
| 2. Menarche is at the age of 10 to 14. |
2. Menopause is at the age of 45 to 50. |
| 3. Menarche begins with secretion of FSH and LH. |
3. Menopause is caused due to decline of FSH and LH secretion. |
| 4. Menarche is the beginning of the reproductive period. |
4. Menopause is the end of the reproductive period. |
Question 6.
Proliferative Phase and Secretory Phase.
Answer:
| Proliferative Phase |
Secretory Phase |
| 1. Proliferative phase begins with the repair of endometrium. |
1. Secretory phase begins with ovulation. |
| 2. Time required for proliferative phase is 5th to 13th day of menstrual cycle. |
2. Time required for secretory phase is 15th to 28th day of menstrual cycle. |
| 3. Proliferative phase always ends with ovulation. |
3. Secretory phase ends with menstruation if egg is not fertilized. It continues further if egg is fertilized. |
| 4. Proliferative phase is in uterus which coincides . with follicular phase in ovary during which there is formation of Graafian follicle. |
4. Secretory phase is in uterus which coincides with luteal phase in ovary during which there is formation of corpus luteum. |
| 5. Proliferative phase is controlled by FSH from anterior pituitary. |
5. Secretory phase is controlled by LH from anterior pituitary. |
| 6. Hormone estrogen is secreted during this phase. |
6. Hormone progesterone is secreted during this phase. |
| 7. It causes the development of blood vessels and thickening of endometrium of uterus. |
7. It causes further thickening and secretory activity of the glands of endometrium of uterus. |
Question 7.
Spermatogenesis and Oogenesis.
Answer:
| Spermatogenesis |
Oogenesis |
| 1. Spermatogenesis takes place in testis in mature and fertile males. |
1. Oogenesis takes place in ovaries in mature and fertile females. |
| 2. From one spermatogonium four haploid sperms are formed during spermatogenesis. |
2. From one oogonium one haploid ovum and a polar body is formed during oogenesis. |
| 3. Spermatid developed undergoes metamorphosis in the process of spermiogenesis. |
3. There is no such process of metamorphosis in oogenesis. |
| 4. Spermatid development takes place which later becomes a functional sperm. |
4. Ootid development does not take place during oogenesis. It develops only after fertilization. |
| 5. Spermatogonia, primary and secondary spermatocytes and spermatid are the stages of sperms formed during spermatogenesis. |
5. Oogonia, primary and secondary oocytes are the stages formed during oogenesis. Ootid formation occurs only after fertilization. |
Question 8.
Zona pellucida and Corona radiata.
Answer:
| Zona pellucida |
Corona radiata |
| 1. Zona pellucida is inner, thin and transparent layer surrounding the secondary oocyte. |
1. Corona radiata is the outer thick layer surrounding the secondary oocyte. |
| 2. Zona pellucida is a non-cellular layer. |
2. Corona radiata is a cellular layer. |
| 3. Zona pellucida is secreted by the ovum itself. |
3. Corona radiata is formed by follicular cells which are glued together by hyaluronic acid. |
| 4. Zona pellucida is retained for more time after fertilization till the ovum gets implanted in the uterus. |
4. Corona radiata is retained till the ovum gets fertilized. |
| 5. Zona pellucida is digested by zona lysine or acrosin at the time of fertilization. |
5. Corona radiata is digested by hyaluronidase enzyme at the time of fertilization. |
Question 9.
Morula and Blastula.
Answer:
| Morula |
Blastula |
| 1. Morula is the embryonic stage formed after the completion of cleavage. |
1. Blastula is the embryonic stage formed after the completion of blastulation. |
| 2. Morula is formed 4 to 6 days after the fertilization. |
2. Blastula is formed 6 to 7 days after the fertilization. |
| 3. Morula consists of 16 cells. |
3. Blastula consists of more than 64 cells. |
| 4. Morula is solid ball of cells. |
4. Blastula is a hollow ball of cells. |
| 5. Morula stage is passed in fallopian tube, once it reaches uterus, it starts developing into the next stage. |
5. Blastula after reaching the uterus is implanted on the wall of uterus. |
| 6. Morula does not have any distinction of its inner cell structure. |
6. Blastula has a blastocoel, trophoblast and inner cell mass. |
Question 10.
Blastula and Gastrula
OR
Give two differences between blastula and gastrula.
Answer:
| Blastula |
Gastrula |
| 1. Blastula is formed from morula on 7th day after fertilization. |
1. Gastrula is formed from blastula 15 days after fertilization. |
| 2. Blastula has a blastocoel. |
2. Gastrula has a gastrocoel or archenteron. |
| 3. Blastula is produced by the process of blastulation. |
3. Gastrula is produced by the process of gastrulation. |
| 4. Blastula undergoes implantation followed by gastrulation. |
4. Gastrula undergoes morphogenesis and then forms germs layers. |
| 5. During blastula formation there is no movement of cells. |
5. Gastrula formation results from the morphogenetic movement of cells. |
Give reasons
Question 1.
Testes are located outside the body cavity in scrotal sacs.
Answer:
- During early foetal life, the testes develop in the lumbar region of the abdominal cavity just below the kidney but during seventh month of development, they descend permanently into the respective scrotal sacs through a passage called inguinal canal.
- For the development of the sperm, lesser temperature than the body temperature is required.
- If the testes remain in the abdominal cavity, then the sperm production does not take place.
- This may result in impotency. Therefore, testes are located outside the body cavity.
Question 2.
Urethra is also called urinogenital duct in males.
Answer:
- Urinogenital duct means common duct for urine and the genital products.
- In males, the penis lodges urethra throughout its entire length, through which urine as well as semen are given out of the body during urination or copulation.
- Since the urethra carries both urine and semen, it is called urinogenital duct.
Question 3.
Proliferative phase is also called follicular phase.
Answer:
- Proliferative phase means there is proliferation of endometrial cells in the uterus. Follicular means there is growth of ovarian follicles in the ovaries. Both these phases are simultaneous.
- The follicular phase of ovaries is due to effect of FSH from adenohypophysis.
- The ovaries follicles grow due to FSH and start secreting estrogen.
- This estrogen from ovaries bring proliferative effect on the uterus.
Question 4.
Missing of menses is the first indication of pregnancy.
Answer:
- Menstruation occurs if there is no fertilization of ovum.
- The endometrium of uterus along with unfertilized egg is given out in the form of menstrual flow.
- The sloughing off uterine endometrium takes place due to degeneration of corpus luteum.
- In the absence of functional corpus luteum progesterone levels fall down. However, if the ovum is fertilized, the corpus luteum is maintained and it secretes progesterone which maintains the uterine endometrium. In such case, further growth of ovarian follicles and ovulation remains suspended and woman is said to be pregnant.
- Endometrial wall of uterus now thickens and helps in the growth of placenta. Thus during pregnancy, menses will not take place.
Question 5.
Progesterone is called pregnancy hormone.
Answer:
- Progesterone is secreted from corpus luteum which is formed from empty ovarian follicle after the ovulation.
- Progesterone has the capacity to maintain pregnancy.
- It acts on uterine endometrium and causes it to proliferate and develop in thickness.
- Corpus luteum keeps on secreting progesterone till the placenta takes up the function of secreting the same.
Question 6.
Human female has restricted reproductive life.
Answer:
- In human female, the reproductive period is about 30 – 33 years.
- There is menarche at the age of about 13 and menopause at the age of 45-50.
- During this span of 30 years, ovaries secrete sex hormones like estrogen and progesterone. After menopause this secretion is suspended.
- Due to changes in hormonal level, human females cannot produce eggs later. Moreover, eggs in her ovaries are utilized by the age of 45.
- Human female, therefore, has restricted reproductive period.
Question 7.
Zona pellucid is retained for sometime after fertilization.
Answer:
- Fertilization of the ovum takes place in fallopian tube where it starts cleavages immediately.
- Zona pellucida which remains on the surface of the ovum prevents the implantation of the blastocyst at an abnormal site such as fallopian tube.
- Zona pellucida keeps the sticky and phagocytic trophoblast cells unexposed till the ovum reaches the uterine lumen.
- Zona pellucida also protects the ovum. Therefore zona pellucida is retained for some time after fertilization.
Question 8.
The acrosome of sperm secretes an enzyme called hyaluronidase at the time of fertilization.
Answer:
- The enzyme hyaluronidase secreted by acrosome of sperm dissolves the membranous covering of the ovum to facilitate the entry of sperm into the ovum.
- It is a lytic enzyme causing lysis of egg membrane.
- Owing to this, the acrosome of sperm secretes an enzyme called hyaluronidase at the time of fertilization.
Question 9.
The middle part of the human sperm is characterized by the presence of a number of mitochondria.
Answer:
- Mitochondria provide energy required by sperms for their agile movement.
- The agile movement of sperms helps them to reach the vicinity of the ovum at the time of fertilization.
- Owing to this, the middle part of the human sperm is characterised by the presence of a number of mitochondria.
Question 10.
The size of morula remains almost same as that of ovum.
Answer:
- The layer zone pellucida is retained around the embryo and thus, there is no change in the overall size from zygote to morula.
- This layer is important because it prevents the implantation of the blastocyst at an abnormal site such as fallopian tube.
- Though the number of blastomeres increase, the size of morula remains almost same as that of ovum till it reaches the uterus by the end of the day 4.
Question 11.
Placenta serves as the nutritive, respiratory and excretory organ of the embryo.
Answer:
- Between the foetus and mother there is exchange of several materials. Food in the form of glucose, amino acids, simple proteins, lipids, mineral, salts, vitamins and hormones, antibodies, etc. is sent to foetus by maternal circulation.
- Oxygen from mother’s blood is also given to the foetus.
- The foetal metabolic wastes such as carbon dioxide, urea and water pass from foetus into the maternal blood.
- This exchange takes place through the placenta.
- In the placenta, foetal blood comes very close to maternal blood to permit these exchanges. Therefore placenta is said to serve as the nutritive, respiratory and excretory organ of the embryo.
Write short notes
Question 1.
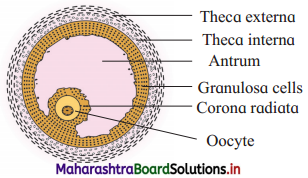
Graafian follicle.
Answer:
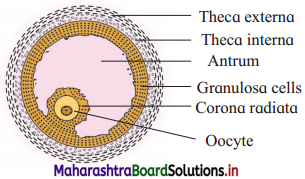
- Graafian follicle is a mature ovarian follicle.
- There are following protective layers on the Graffian follicle : The outermost protective and fibrous covering, theca externa. Theca interna is the next layer which can secrete hormone estrogen.
- Next to theca interna, there is membrana granulosa which forms discus proligerous and the corona radiata layer.
- Graafian follicle contains an eccentric secondary oocyte. The oocyte is surrounded by a vitelline membrane which produces zona pellucida layer.
- In the centre there is antrum which is filled with liquor folliculi fluid.
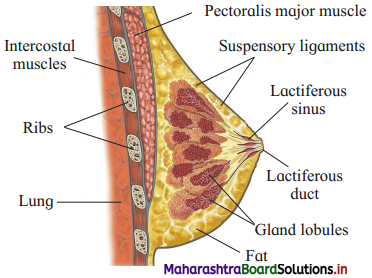
Question 2.
Mammary glands.
Answer:
- Mammary glands are accessory organs of female reproductive system. These glands are essential for lactation after parturition.
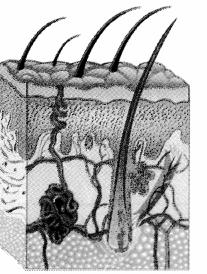
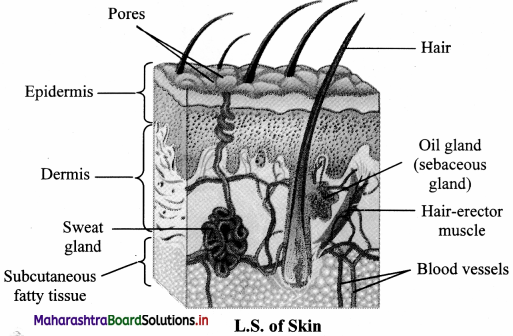
- They are modified sweat glands present in the subcutaneous tissue of the anterior thorax. They are in the pectoral region in the location between 2nd to 6th rib.
- Each mammary gland consists of fatty connective tissue and many lactiferous ducts.
- Each breast has glandular tissue which is divided into 15-20 irregularly shaped mammary lobes. Each lobe has an alveolar glands and lactiferous duct.
- Milk is secreted by alveolar glands and it is stored in the lumen of alveoli. The alveoli open into mammary tubules and these in turn forms a mammary duct.
- All the lactiferous ducts converge towards the nipple.
- Nipple is surrounded by a dark brown coloured and circular area of the skin called areola.

Question 3.
Structure of sperm.
Answer:
- Sperm is microscopic, elongated haploid motile male gamete produced by spermatogenesis.
- It measures to about 0.055 mm or 60y in length.
- The sperm consists of head, neck, middle piece and tail.
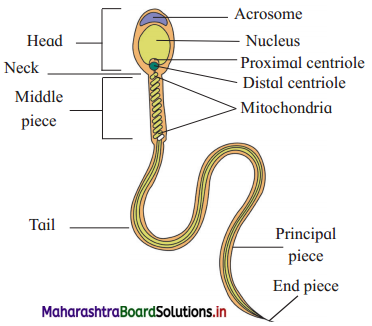
Head:
- Head is the main part which is flat and oval and has a large nucleus and an acrosome.
- Acrosome is formed from Golgi complex. It secretes enzyme hyaluronidase which helps in penetration of the egg during fertilization.
- The acrosome and anterior half of nucleus is covered by a fibrillar sheath.
Neck : Neck is short region having two centrioles.
- The proximal centriole plays a role in first cleavage of zygote.
- The axial filament of the sperm is formed by the distal centriole.
Middle piece:
- Middle piece acts as a power house for sperm.
- It bears many spirally coiled mitochondria or Nebenkern around the axial filament.
- The mitochondria supply energy for the sperm to swim in the female genital tract with a speed of about 1.5 to 3 mm per minute.
- Posterior half of nucleus, neck and middle piece of sperm are covered by a sheath.
Tail:
- The tail is formed of cytoplasm and is long, slender and tapering structure.
- The axial filament is a fine thread-like structure that arises from the distal centriole and traverses the middle piece and tail.
- Nine accessory fibres are present surrounding the two central longitudinal axial filaments.
- Tail lashes and helps the spermatozoa to swim.
Question 4.
Structure of secondary oocyte.
Answer:
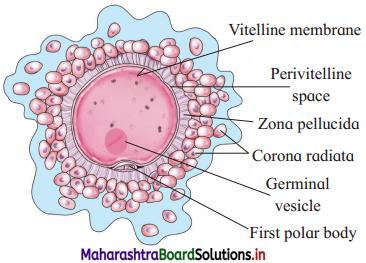
- The unfertilized egg released through ovary at the time of ovulation is a secondary oocyte.
- It is rounded, non-motile and haploid, non- cleidoic and microlecithal female gamete.
- The size is approximately 0.1 mm (100 microns).
- It has abundant cytoplasm called ooplasm which contains a large eccentric and prominent nucleus called germinal vesicle.
- Centrioles are absent in secondary oocyte.
- Various coverings seen around the oocyte are (i) vitelline membrane (ii) zona pellucida (iii) Corona radiata.
- The cells are glued together with hyaluronic acid. Between vitelline membrane and zona pellucida, there is perivitelline space which lodges first polar body. This end is called 5 animal pole and the opposite is called vegetal pole.
Question 5.
Implantation
Answer:
- Implantation is the process by which the blastocyst is embedded into the endometrium of uterus in the fundus region.
- The implantation starts 7 days after fertilization and completed by the end of 10th day.
- The trophoblast cells of blastocyst at the embryonal knob can stick to the uterine endometrium. The trophoblast layer then divides into inner cytotrophoblast and outer syncytiotrophoblast due to contact with endometrial cells.
- Cytotrophoblast is the inner layer whose cells retain their cell boundaries.
- Syncytiotrophoblast is the outer layer of cells without plasma membrane. The cells of syncytiotrophoblast appear multinucleate. This layer projects invasively into the endometrium and destroys endometrial cells by releasing lytic enzymes. Due to this blastocyst is buried deeply in the endometrium.
Question 6.
Fate of three germinal layers.
Answer:
Fate of germinal layers : The embryo after gastrulation develops the three germ layers, viz., ectoderm, mesoderm and endoderm. Later a process of histogenesis starts which leads to the development of different tissues and organs.
(i) Fate of ectoderm : Following tissues, structures and organs develop from the ectoderm : Epidermis of the skin, epidermal derivatives such as
- hair and nails
- sweat glands
- conjunctiva
- cornea
- lens
- retina
- internal and external ear
- enamel of teeth
- nasal cavity
- adrenal medulla
- stomodaeum and proctodaeum
- neurohypophysis and
- entire nervous system.
(ii) Fate of mesoderm : The mesoderm forms the following derivatives:
- All types of muscles
- connective tissue
- dermis of skin
- adrenal cortex
- kidney
- circulatory system
- heart
- blood vessels
- blood
- lymphatic vessels
- middle ear and
- dentine of teeth.
(iii) Fate of endoderm : The following organs develop from the endoderm:
- Epithelium of gut from pharynx to colon
- glands of stomach and intestine
- tongue and tonsils
- lungs, trachea, bronchi, larynx, etc.
- urinary bladder, vestibule and vagina
- liver and pancreas
- adenohypophysis
- thymus, thyroid and parathyroid
- eustachian tube
- epithelium of urethra and associated glands.
Question 7.
Placenta.
Answer:
- Placenta is a temporary organ derived from the tissues of the foetus as well as mother.
- Human placenta is called chorionic placenta as it is made up of chorion which is an extra-embryonic membrane.
- Blood vessels from the allantois vascularize the placenta. Branching villi emerge from the chorion and penetrate in the corresponding pits which are located in the uterine wall.
- There are two parts of placenta, viz. foetal placenta and maternal placenta.
- Foetal placenta is formed of chorionic villi.
- Maternal placenta is formed of uterine wall which is in intimate contact with the chorionic villi.
- Chorionic villi receive the blood from the embryo by umbilical artery. Umbilical vein returns the blood back to the embryo.
- Human placenta is said to be haemochorial because a part of placenta is from foetus which has chorionic villi. The other highly vascularized part is from uterine wall of mother. Thus foetal and maternal placenta together is called haemocorial placenta.
Question 8.
Intratuterine devices (IUDs).
Answer:
- IUDs are plastic or metal objects which act as contraceptive devices. They are placed into the uterus by a doctor or trained nurse.
- E.g. Lippe’s loop, copper releasing IUDs (Cu-T, Cu-7, multiload 375) and hormone releasing IUDs (LNG-20, Progestaert).
- Plastic double ‘S’ loop is called Lippe’s loop which stimulates accumulation of macrophages in the uterine cavity by attracting them. As phagocytosis increases the sperms are destroyed. Thus it acts as a contraceptive.
- Copper releasing IUDs suppress sperm motility and the fertilizing capacity of sperms.
- The hormone releasing IUDs make the : uterus unsuitable for implantation and ; cervix hostile to the sperms.
- Their presence in the uterus acts as a minor irritant and thus makes the ovum to move quickly out of the body.
- However, IUD can cause infection and occasional haemorrhage. It can cause discomfort for woman and may get spontaneously expelled out.
Question 9.
Physiological (Oral) Contraceptive « Devices.
Answer:
- Physiological devices are in the form of oral contraceptive pills or birth control pills. 5 They are hormonal preparations and check ovulation by inhibiting the secretion of follicle stimulating hormone and luteinizing hormone.
- Woman who is using pills does not release ovum at the time of ovulation and therefore conception does not occur.
- Birth control pills have side effects such as nausea, breast tenderness, weight gain and ‘break through’ bleeding, i.e. slight bleeding between the menstrual periods. These health hazards are due to synthetic hormones.
- These pills also alter the quality of cervical J mucus to prevent the entry of sperms.
- The birth control pills contain progesterone and estrogen. Mala-D to be taken daily and Saheli to be taken weekly are two common birth control pills in India. These pills are non-steroidal.
Question 10.
Fate of trophoblast cells of blastocyst.
Answer:
- Trophoblast cells do not form any part of the embryo proper.
- They form ectoderm of the extra-embryonic membrane called chorion.
- Chorion helps in supply of oxygen and nutrients to foetus from mother’s body. CO2 and nitrogenous wastes are collected from foetus and passed in mother’s blood.
- Thus, these cells have an important role in formation of placenta.
Question 11.
Medical Termination of Pregnancy (MTP).
Answer:
- MTP or Medical Termination of Pregnancy is voluntary termination of pregnancy under medical supervision. It is an induced abortion.
- Only during first trimester, MTP is safe for mother’s health.
- Upon amniocentesis examination, if abnormality is detected, usually MTP is performed.
- Government of India has legalized MTP There was MTP Act in 1971, which was later amended in 2017, to prevent its misuse, especially female foeticide should never be done through MTP
- As per MTP Act, the procedure can be done only in first 12 weeks and never after 20 weeks of pregnancy.
Question 12.
Amniocentesis
Answer:
- In amniocentesis, amniotic fluid containing foetal cells is collected using a hollow needle. This needle is inserted into the uterus of pregnant mother, under ultrasound guidance.
- The chromosomes from the foetal cells are sujected to karyotyping. This helps to detect abnormalities in the developing foetus.
- Amniocentesis is misused to determine the sex of the unborn child. This is illegal in India because it results into female foeticide.
- Another risks involved in amniocentesis are miscarriage, needle injury to foetus, leaking amniotic fluid, infection, etc.
- As per MTP Act (1971) the misuse of amniocentesis is curtailed.

Question 13.
ZIFT [Zygote Intra Fallopian Transfer].
Answer:
- If there is a blockage in the fallopian tubes due to which fertilization is prevented, then ZIFT treatment is used.
- The oocyte is removed form woman’s ovary. This oocyte is fertilized outside the body under sterile conditions with the known sperms. This forms zygote. This is In Vitro Fertilization (IVF).
- Later the zygote is transferred in fallopian tube to achieve pregnancy.
Question 14.
GIFT [Gamete Intra Fallopian Transfer].
Answer:
- When the oocyte is collected from donor and transferred into the fallopian tube of another female, the technique is called GIFT. This female provides suitable environment for further development.
- When the entrance or upper segments of the fallopian tubes is blocked, this technique is used.
- Ooocytes and sperms are directly injected into regions of the fallopian tubes. Here fertilization takes place forming a blastocyst. It later enters the uterus for implantation.
- GIFT is successful in only 30 per cent cases.
Question 15.
Sterilization operations.
Answer:
- Sterilization operations are the permanent means for the birth control. These can be performed on both the sexes. Usually these are performed after the couple does not desire another child.
- These surgical interventions block the gamete transport and thus prevents the pregnancy.
- Sterilization operation in males is called vasectomy while in females it is called tubectomy.
- In vasectomy the vas deferens are tied and cut. In tubectomy fallopian tubes are ligated or cut.
Question 16.
Gonorrhoea.
Answer:
(1) Gonorrhoea is a sexually transmitted veneral disease caused by Diplococcus bacterium, Neisseria gonorrhoea.
(2) The incubation period is 2 to 14 days in males and 7 to 21 days in females.
(3) Infection sites are mucous membrane of urino-genital tract, rectum, throat and eye.
(4) Males show following symptoms : Partial blockage of urethra and reproductive ducts, pus from penis, pain and burning sensation : during urination, arthritis, etc.
(5) Symptoms in female include, pelvic inflammation of urinary tract, sterility, arthritis. The children born to affected mother suffer from gonococcal ophthalmia, In girl-child, there is occurrence of gonococcal vulvovaginitis before puberty.
(6) Preventive measures for gonorrhoea are as follows:
- Sexual hygiene
- Use of condom during coitus.
- Sex with unknown partner or multiple partners should be avoided.
(7) Gonorrhoea can be treated with Cefixime which is antibiotic.
Short-answer Questions
Question 1.
What are sexual dimorphic characters? Enlist these characters in human male and female.
Answer:
Sexual dimorphism is the phenomena in which the sexes of the individual can be identified externally. In human beings, even in infancy there is sexual dimorphism, by which one can identify the sex of the infant.
But when the male or female reaches puberty, then secondary sexual characters are developed due to sex hormones. These characters are called sexual dimorphic characters.
(i) Secondary sexual characters in males:
- Presence of beard, Moustache.
- Hair on the Chest, Axillary and Pubic Region.
- Muscular body.
- Enlarged larynx (Adam’s apple).
(ii) Secondary sexual characters in females:
- Breast development.
- Broadening of pelvis.
- High pitched voice.
Question 2.
Describe the duct system that transports the sperms from seminiferous tubules to the exterior.
Answer:
(1) All the seminiferous tubules present in the testis show posterior network of tubules called rete testis. Vasa efferentia are the fine tubules which are 12-20 in number, are seen arising from rete testis. From testis to epididymis, the sperm transport is done by vasa efferentia.
(2) Epididymis has three parts, caput, corpus and cauda epididymis. In this long and highly coiled tube sperms undergo physiological maturation.
(3) Then from here sperms enter into vas deferens, which is a tube that arise from epididymis enters the abdominal cavity. On its course, later it joins the duct of seminal vesicle. Both together form the ejaculatory duct.
(4) Ejaculatory duct passes through the prostate gland and then opens into the urethra. Urethra is a common passage for urine and semen and hence it is also called urinogenital duct.
(5) Urethra passes through penis and opens to the outside by an opening called the urethral meatus or urethral orifice.
(6) Thus sperms are transported through vas deferens into urethra via ejaculatory duct and then to the outside through urethral orifice.
Question 3.
What is semen? Describe the composition of semen.
Answer:
(1) Semen is the viscous, alkaline and milky fluid having pH 7.2 to 7.7 ejaculated during sexual intercourse by male.
(2) A single ejaculation of semen i.e. 2.5 to 4 ml semen contains about 400 millions of sperms.
(3) Semen consists of sperms suspended in secretions of the epididymis and the accessory glands (seminal vesicles, prostate gland and Cowper’s gland). The semen nourishes the sperms by fructose, neutralizes acidity by Ca++, ions and bicarbonates and also activates them for movement due to prostaglandins.
Question 4.
Describe in detail the external genitalia of human female reproductive system.
Answer:
The external genital organs of female are located external to the vagina. They have collective name, ‘vulva’ or pudendum. Following are the parts of vulva.
(1) Labia majora : Labia majora are homologous to scrotum of males. They are two large folds which form the boundary of the vulva. They are composed of skin, fibrous tissue and fat. These Eire prominent and longitudinal folds on right and left sides of the vestibule.
(2) Labia minora : Smaller and thinner lip-like folds located just medially are labia minora. Posteriorly the labia minora are fused together to form the fourchette.
(3) Mons veneris : Mons veneris is fleshy elevation above the labia majora.
(4) Clitoris : It is present at the anterior end of the labia minora. It shows the presence of erectile tissues.
(5) Vestibule : Vestibule is a median vertical depression of vulva enclosing vagina and urethral opening.
(6) Hymen : Hymen is a thin layer of mucous membrane which partially occludes the opening of the vagina.
(7) Vestibular glands:
- Vestibular glands or Bartholin’s glands are homologous to the Cowper’s glands of the male.
- These are paired glands situated on either side of the vaginal opening, secreting lubricating fluid.
Question 5.
How is puberty attained in females? Will a female normally remain reproductively capable even after age 50? If not then what makes her incapable?
Answer:
(1) Puberty is achieved due to gonadotropins such as FSH and LH secreted by the anterior pituitary. These hormones stimulate the ovaries. The ovaries in turn produce estrogen and progesterone, which brings about secondary sexual characters in female. Thus she attains the puberty. The beginning of menstrual cycle or menarche takes place due to these hormonal changes at about 10 to 14 years.
(2) But the women do not remain reproductively active after the age of 50 due to hormonal imbalance. This is called menopause or cessation of reproductive cycles. Absence of enough gonadotropins and unresponsive ovarian cells cause menopause at 45 to 50 years of age.
Question 6.
Why is menstruation painful in some women?
Answer:
- The menstruation is painful in some women as the muscles in the uterus contract or tighten.
- Women who experience painful periods can have higher levels of prostaglandins, a natural body chemical that causes contractions of the uterus and blood vessels.
- Some women have a build-up of prostaglandins which means they experience stronger contractions and therefore due to spasmodic pain in some women menstruation is more painful.
- Endometrial sloughing that takes at the time of menstruation also causes painful discomfort.
Question 7.
Why is it said that consumption of mother’s milk is safety for the newborn?
Answer:
Consumption of mother’s milk is safety for the newborn because of the following reasons:
- Mother’s milk is the perfect food for babies in the first months of their lives. With the exception of vitamin D, it contains all the nutrients an infant needs.
- Mother’s milk supplies antibodies [IgA] that protect the baby’s body organs from infections. Mother’s milk provides immunity and also help in maturation of the infant’s immune system which are lacking in ordinary milk. Natural acquired passive immunity is obtained only through mother’s milk.
- Feeding of mother’s milk reduces the risk of overweight and obesity during childhood.
- It also creates the bond between mother and child.
Question 8.
Which hygienic practices should be followed by the female during menstruation ?
Answer:
The following personal hygienic practices should be followed by the female during menstruation:
- Keeping the pubic area clean.
- Changing the sanitary napkin every 4-5 hours.
- Reducing risk of infections by maintaining hygiene.
- Proper disposal of soiled sanitary napkin.
- Not to use damp and dirty clothes which can cause infections and bad odour. A sanitary napkin which is not changed in time can act as a perfect environment for rapid growth of infectious bacteria.
Question 9.
How can the goals of RCH be achieved?
Answer:
The goads of RCH can be achieved by the following ways:
- Sex education in schools is introduced. Proper and scientific information about sexual organs and safe sexual acts should be given to students. They should be made aware of sexually transmitted diseases (STD, AIDS), and problems related to adolescence.
- Audio-visual and the print media should be used by government and non-government organisations for creating awareness about reproductive health.
- Younger generation should be educated about family planning measures, pre-natal and post-natal care of women and care of infant with knowledge about importance of breastfeeding.
- Awareness should be spread about problems arising due to uncontrolled population growth, sex abuse and sex related crimes. Necessary steps to prevent these to be taken.
- Statutory ban on amniocentesis for sex determination is practised. This should be known by all.
- Details of child immunization programmes should be understood.
- New parents should get the training for new born care so that infant and maternal mortality rate can be reduced.
Question 10.
How do addictions like smoking, alcoholism and drug abuse contribute in causing infertility in men?
Answer:
- Tobacco, marijuana and other drugs, smoking may cause infertility in both men and women.
- Nicotine blocks the production of sperm and decreases the size of testicles.
- Alcoholism by men interferes with the synthesis of testosterone and has an impact on sperm count.
- Use of cocaine or marijuana may temporarily reduce the number and quality of sperm.
Question 11.
Jayesh, a young married man of 26 years is suffering from T.B. for the last 2 years. He and his wife are desirous of a child but unable to have one, what could be the possible reason? Explain.
Answer:
Jayesh, though young, is suffering from TB for last 2 years. His wife is unable to conceive the child may be due to following reasons:
- Tuberculosis disrupts sexual and reproductive function in patients.
- Moreover T.B. patients have to take not less than 4 anti-TB drugs simultaneously for a long time.
- These are basically a very high dose antibiotics which may hamper formation of sperms. In this way the anti-tuberculosis drugs may negatively influence on sexual function.
- Pulmonary TB patient shows, deterioration of all parameters of copulatory act, from sexual desire to orgasm and thus the couple is unable to conceive.
- Infertility is one of the most common symptoms of genital tuberculosis.
Question 12.
Neeta is 45 years old and the doctor advised her not to go for such a late pregnancy. She however wants to be the biological mother of a child without herself getting pregnant. Is this possible and how?
Answer:
(1) Neeta being 45 years old, she is approaching menopause. Therefore, she will be advised by the doctor to take the help of the modern remedial technique called surrogacy.
(2) In this technique the embryo is formed using intended father’s sperm and intended mother’s egg by In Vitro Fertilization (IVF) technique and then that embryo is implanted in a surrogate mother, sometimes called a gestational carrier.
(3) In surrogacy there is legal arrangement where the surrogate mother agrees to bear child for a couple. Remains pregnant with all the care and nourishment. Later she delivers a baby and hands it over to biological mother.
Chart based /Table based questions
Question 1.
Complete the following chart and rewrite
| Female Reproductive Organs |
Homology to Male Reproductive Organs |
| 1. Labia majora |
————– |
| 2. ————- |
Bulbourethral glands/ Cowper’s gland |
| 3. Clitoris |
————— |
Answer:
| Female Reproductive Organs |
Homology to Male Reproductive Organs |
| 1. Labia majora |
Scrotum |
| 2. Bartholin’s gland/ Vestibular gland |
Bulbourethral glands/ Cowper’s gland |
| 3. Clitoris |
Penis |
Question 2.
| Hormones |
Functions |
| 1. Testosterone |
————– |
| 2. ————- |
Stimulates contractions uterine during parturition |
| 3. Progesterone |
————— |
Answer:
| Hormones |
Functions |
| 1. Testosterone |
Stimulates spermatogenesis |
| 2. Oxytocin |
Stimulates contractions uterine during parturition |
| 3. Progesterone |
Maintain endometrium of uterus during secretory phase and gestation. |
Question 3.
Complete the following chart and rewrite
| Hormones |
Functions |
| 1. Rapid regeneration of endometrium and maturation of Graafian follicle |
————– |
| 2. Secretion of endometrial glands and increased secretion of progesterone |
————– |
| 3. Breakdown of endometrium in absence of fertilization |
————- |
Answer:
| Hormones |
Functions |
| 1. Rapid regeneration of endometrium and maturation of Graafian follicle |
Proliferative phase / Follicular phase |
| 2. Secretion of endometrial glands and increased secretion of progesterone |
Secretory phase / Luteal phase |
| 3. Breakdown of endometrium in absence of fertilization |
Menstrual phase |
Diagram based questions
Question 1.
Sketch and label Human male reproductive system.
Answer:
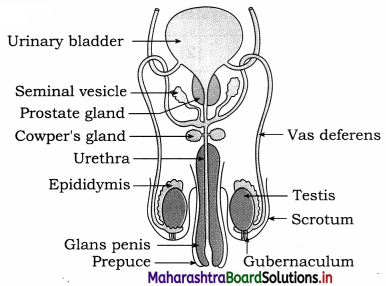

Question 2.
Label the given male reproductive system you have studied.
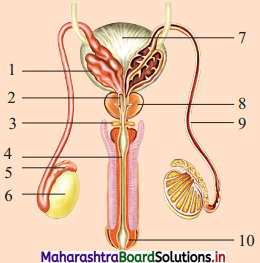
Answer:
- Seminal vesicle
- Ejaculatory duct
- Cowper’s glands
- Urethra
- Epididymis
- Testis
- Urinary bladder
- Prostate gland
- Vas deferens
- Penis
Question 3.
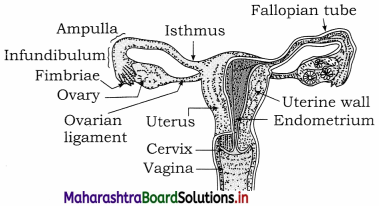
Sketch and label human female reproductive system.
Answer:
Question 4.
Give labels to given diagram of female reproductive system.
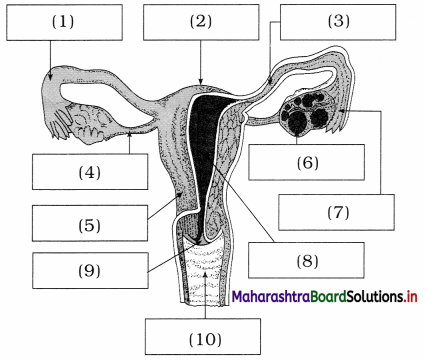
Answer:
- Fallopian tube
- Fundus of Uterus
- Ampulla of fallopian tube
- Ovarian ligament
- Uterus
- Ovary
- Infundibulum with fimbriae
- Endometrium of uterus
- Cervix
- Vagina
Question 5.
Sketch and label Seminiferous tubules as seen in T.S. of testis.
Answer:
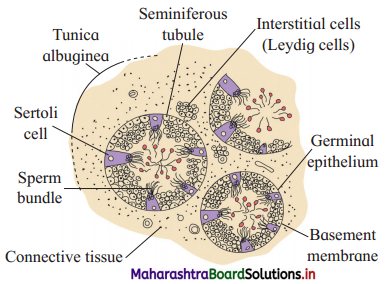
Question 6.
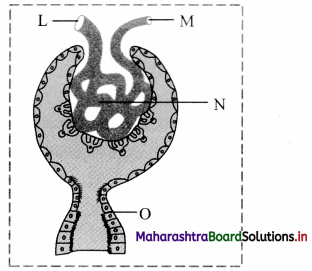
Identify ‘A’ and ‘B’ in the diagram below and mention their functions.
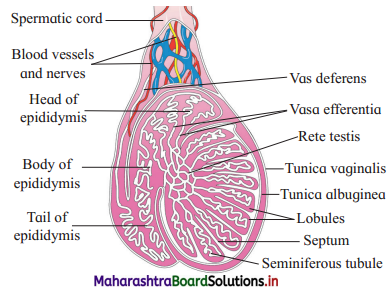
Answer:
(1) A : Seminiferous tubule
Function : Seminiferous tubules produce sperms by spermatogenesis.
(2) B : Vas deferens
Function : Vas deferens carry sperm from epididymis to ejaculatory duct.
Question 7.
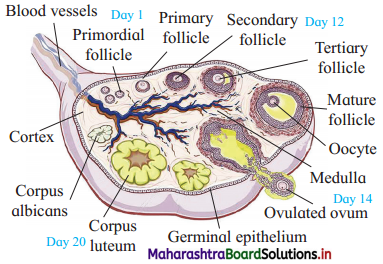
Sketch and label – T.S. of ovary.
Answer:
Question 8.
Sketch and label sectional view of mammary gland.
Answer:
Question 9.
Sketch and label – Graafian follicle.
Answer:
Question 10.
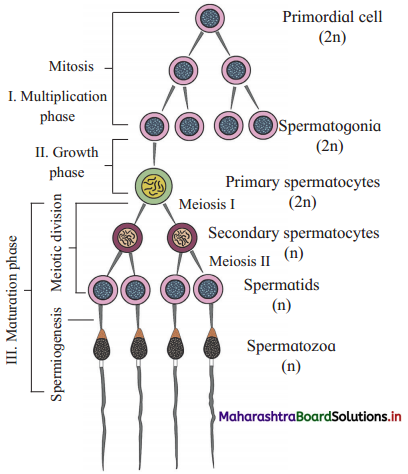
Sketch and label – Process spermatogenesis.
Answer:
Question 11.
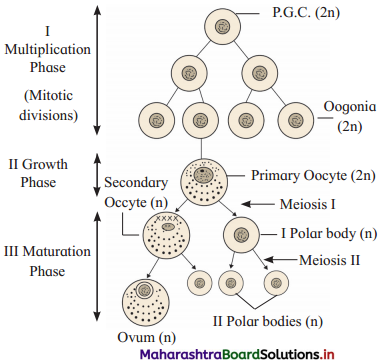
Sketch and label process of oogenesis.
Answer:
Question 12.
Give the name and functions of ‘A’ and ‘B’ from the diagram given below
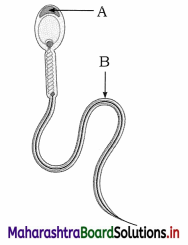
Answer:
(1) A is acrosome.
Function of acrosome : Acrosome produces lytic enzyme, hyalourinidase and thus helps in the penetration of the egg during fertilization.
(2) B is tail of the human sperm.
Function of tail : Tail lashes continuously and helps the movement of the sperm in the female genital tract.
Question 13.
The diagram represents a surgical sterilization method in males. Study the same and answer the questions that follow
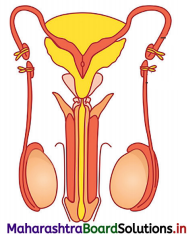
- Give the name of the surgical method represented in the diagram.
- Which part is ligated or cut.
- Name the corresponding surgical method conducted in females.
- Name the part which is ligated in females and why?
Answer:
- Vasectomy
- Vas deferens
- Tubectomy
- Fallopian tubes are ligated so that the egg may not meet with the sperms.
Question 14.
Given below is the figure of an important structure developed during pregnancy.
- Name the structure and its type.
- Identify ‘A’. In which technique it is used.
- Identify ‘B’ What is its function?
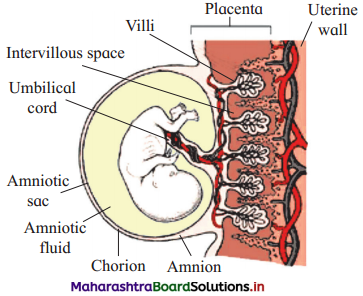
Answer:
(1) The given figure is placenta. The type of placenta in humans is haemochorial placenta.
(2) A is amnio tic fluid. Amnio tic fluid is withdrawn in amniocentesis technique. From this fluid foetal cells can be obtained, which are examined for any chromosomal abnormality by karyotyping.
(3) B is umbilical cord. This is the connection between placenta of mother and growing foetus. Through the umbilical cord, foetus gets nutrition and oxygen. Nitrogenous wastes and carbon dioxide is collected from foetus and brought into maternal circulation.
Question 15.
The diagram given below is that of a intra-uterine contraceptive device. Study the same and then answer the questions that follows:

- Give the name of intra-uterine contraceptive device shown in the diagram.
- What is its mode of action?
Answer:
- The Intra-uterine contraceptive device shown in the diagram is Lippes loop.
- It is a plastic double ‘S’ loop. It attracts the macrophages stimulating them to accumulate in the uterine cavity. Macrophages increase phagocytosis of sperms within the uterus and acts as a contraceptive.
Question 16.
Identify A in the given diagram. Write the function of the same.
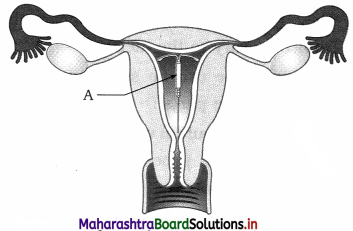
Answer:
A in the above diagram is intrauterine device or IUD. It is a contraceptive device inserted in the uterus of woman. This is a hormone releasing IUD. It acts as a mechanical means of contraception and avoids pregnancy.

Long answer questions
Question 1.
With the help of diagrammatic representation, explain the process of spermatogenesis.
Answer:
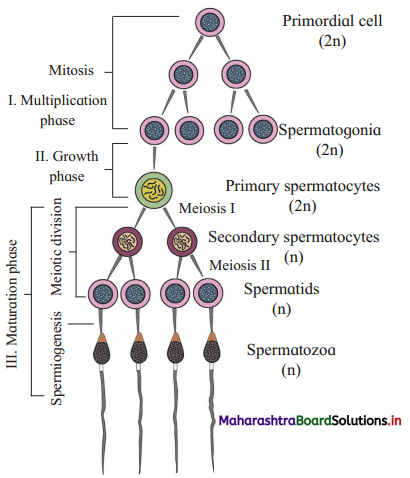
(1) The process of spermatogenesis takes place in the male gonads or testis. The cells of germinal epithelium that line the seminiferous tubules undergo spermatogenesis.
(2) Primordial germ cells or germinal cells pass through three phases, viz. phase of multiplication, phase of growth and phase of maturation.
- Multiplication phase : Primordial germ cells undergo mitotic divisions to produce many diploid (2n) spermatogonia.
- Growth phase : Spermatogonium accumulates nutrients and grows in size, giving rise to primary spermatocyte (2n).
- Maturation phase : The primary spermatocyte undergoes first meiotic division or maturation division. Exchange of genetic material occurs between homologous chromosomes in each spermatocyte.
(3) The meiotic division gives rise to secondary spermatocyte which is haploid (n). At the end of first meiotic division two secondary spermatocytes are formed while at the end of second meiotic division four haploid spermatids are formed.
(4) Spermatids are non-motile. They undergo spermiogenesis and form motile spermatozoan (sperm).
(5) The changes taking place during spermiogeneis are as follows:
- Increase in length.
- Formation of proximal and distal centriole.
- Distal centriole forms the axial filament.
- Mitochondria become spirally coiled.
- Acrosome is formed from Golgi complex.
Question 2.
What is oogenesis? Describe it briefly.
Answer:
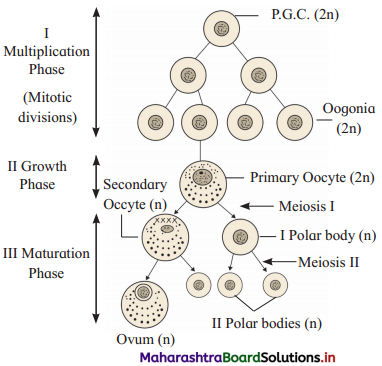
1. Oogenesis is the process of formation of the haploid female gamete, i.e. ovum.
2. The process of oogenesis takes place in the follicular cells inside the ovaries. The germinal epithelium cells undergo oogenesis.
3. They pass through three phases, viz. phase of multiplication, phase of growth and phase of maturation at the time of oogenesis.
- Multiplication phase : Germinal cells undergo mitotic divisions and produce large number of diploid (2n) oogonia. Oogonia are present in the ovaries of female even before she is born.
- Growth phase : During puberty changes, the FSH from pituitary makes one oogonium to develop at a time. The growth takes place as the follicle matures and larger primary oocyte (2n) is produced inside the Graafian follicle.
- Maturation phase : The primary oocyte undergoes first meiotic division. There are equal nuclear divisions during meiosis but the cytoplasm is unequally divided.
4. By the end of first meiotic division, larger haploid secondary oocyte and smaller haploid polar body are produced. Since the embryo develops from the egg, there is provision for more food in the secondary oocyte.
(5) The second meiotic division takes place in the secondary oocyte and polar body. But this division is arrested during metaphase.
(6) The secondary oocyte is released from the ovary in the process of ovulation. Remaining division takes place if and only if ovum is fertilized.
(7) The division is unequal and form functional female gamete or ovum at the time of fertilization.
Question 3.
What is gastrulation? What are the changes that are brought about by gastrulation?
Answer:
(1) Gastrulation : The process of formation of three germ layers by morphogenetic movements and rearrangements of the cells in blastula leading to the formation of gastrula is known as gastrulation.
(2) Cells on the free end of inner cell mass called hypoblasts (primitive endoderm) become flat, divide and grow towards the blastocoel to form endoderm.
(3) Endodermal cells grow within the blastocoel to form a Yolk sac.
(4) The remaining cell of the inner cell mass, in contact with cells of Rauber are called epiblasts (primary ectoderm) which further differentiate to form ectoderm.
(5) Cells of ectoderm divide and re-divide and move in such a way that they enclose the amniotic cavity. The floor of this cavity has the embryonal disc while roof is lined by amniogenic cells. Amnion is an extra embryonic membrane that surrounds and protects the embryo.
(6) Actual gastrulation occurs about days after fertilization.
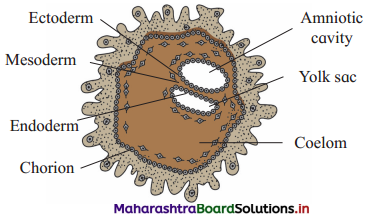
(7) Trilaminar embryonic disc begins with the formation of primitive streak and a shallow groove on the surface called primitive groove. From the site of primitive streak, a third layer of cells called mesoderm extends between ectoderm and endoderm. Anterior end of the primitive groove communicates with yolk sac by an aperture called blastopore (future anus).
(8) The embryonal knob thus finally differentiate into three layers – ectoderm, mesoderm and endoderm.
Question 4.
Explain the major changes taking place during the three trimesters of pregnancy in woman.
Answer:
The pregnancy period of approximately nine months (280 days) is divided into three trimesters of three months each.
1. First Trimester : (From fertilization to 12th week)
- During first trimester there are radical changes in the body of mother as well as in the embryo.
- The embryo receives nutrients in the first 2-4 weeks directly from the endometrium.
- It is the main period of organogenesis and the development of body organs.
- By the end of eight weeks, the major structures found in the adult are formed in the embryo in a rudimentary form. It is now called foetus and is about 3 cm long.
- Arms, hands, fingers, feet, toes, CNS, excretory and circulatory system including heart are formed and begins to work.
- Progesterone level becomes high and menstrual cycle is suspended till the end of pregnancy.
- At the end of first trimester foetus is about 7-10 cm long.
- The maternal part of placenta grows, the uterus becomes larger. In this period, the mother experiences morning sickness, (nausea, vomiting, mood swings, etc.)

2. Second Trimester: (From 13th to 26th week)
- The foetus is very active and grows to about 30 cm.
- The uterus grows enough for the pregnancy to become obvious.
- Hormone levels stabilize as hCG declines, the corpus luteum deteriorates and the placenta completely takes over the production of progesterone which maintains the pregnancy.
- Head has hair, eyebrows and eyelashes appear, pinnae are distinct. Baby’s movement can be easily felt by the mother.
- The baby reaches half the size of a new born.
3. Third Trimester: (From 27th week till the parturition)
- Foetus grows to about 50 cm in length and about 3-4 kg in weight.
- As the foetus grows, the uterus expands around it, the mother’s abdominal organs become compressed and displaced, leading to frequent urination, digestive blockages and strain in the back muscles.
- At the end of third trimester the foetus becomes fully developed and ready for parturition.
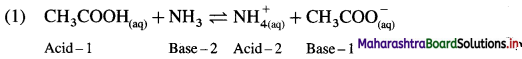
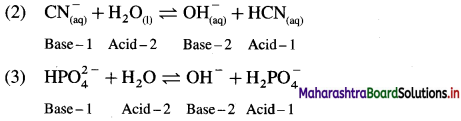
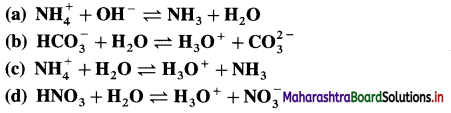
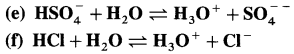
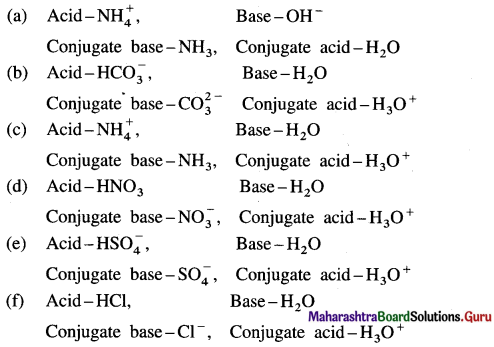

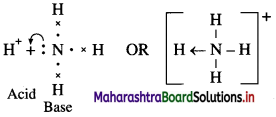
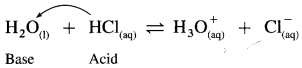
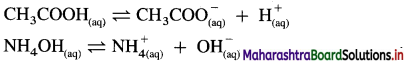
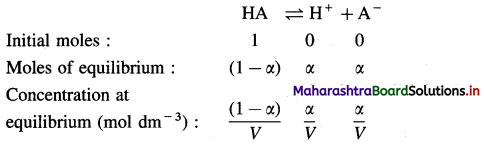
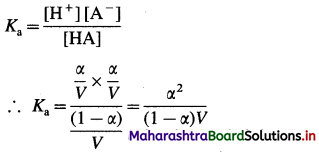
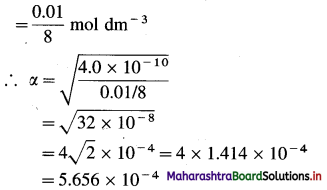
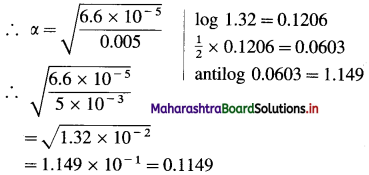
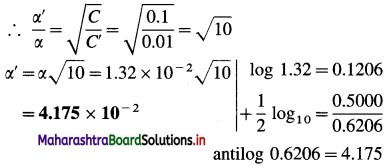
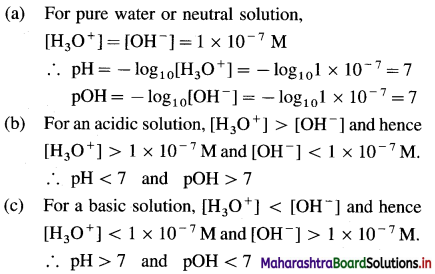
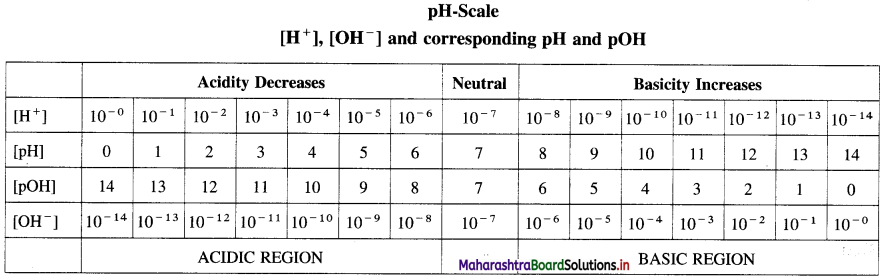
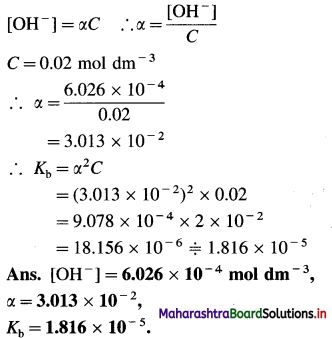
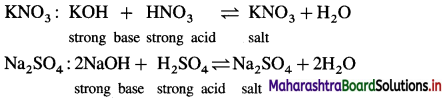
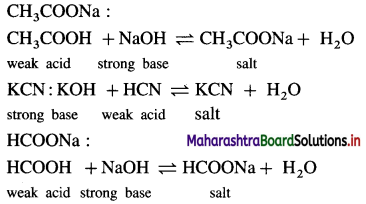
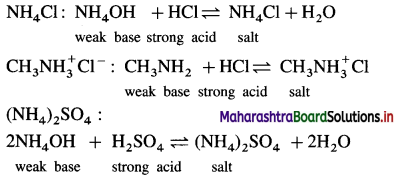
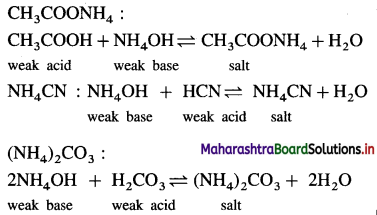
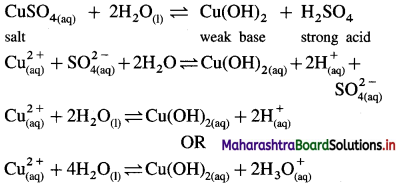
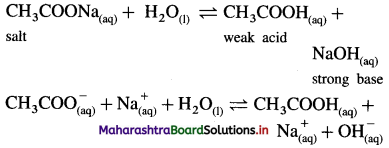
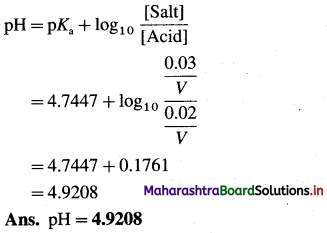
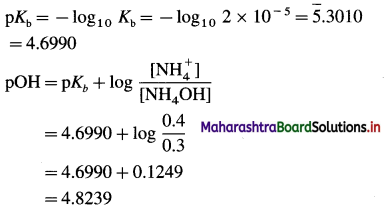


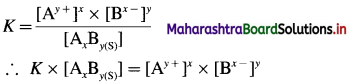
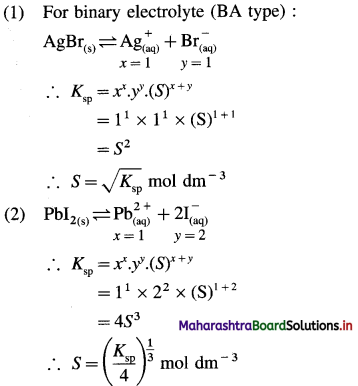
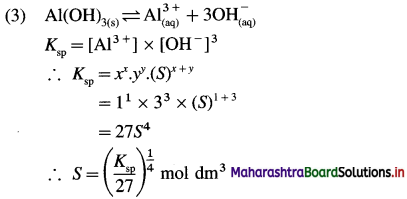
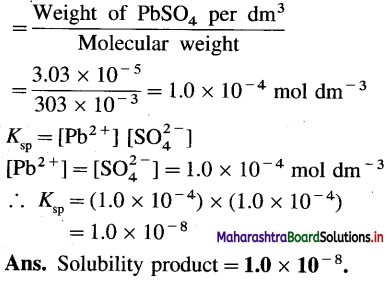
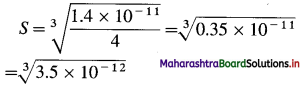
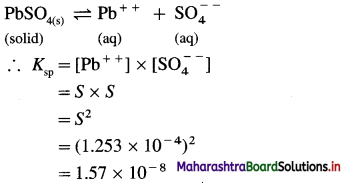
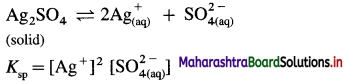



 Ring opening polymerization proceeds by addition of a single monomer unit to the growing chain molecules. It is a step growth polymerization.
Ring opening polymerization proceeds by addition of a single monomer unit to the growing chain molecules. It is a step growth polymerization.













 is used in the preparation of polyester (terylene or dacron).
is used in the preparation of polyester (terylene or dacron).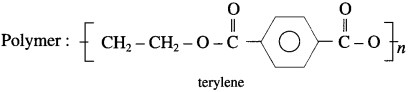
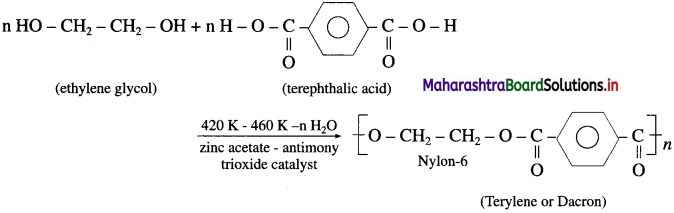
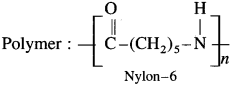
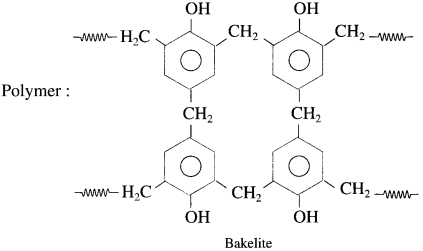
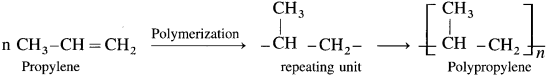
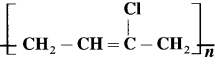
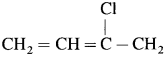
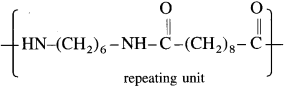


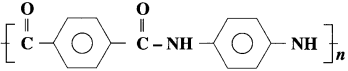
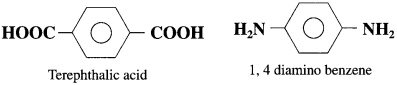
 is the formula of _ Jn
is the formula of _ Jn