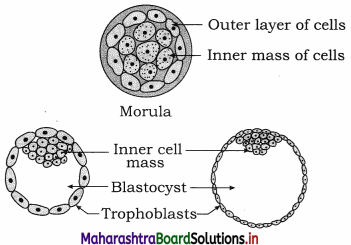
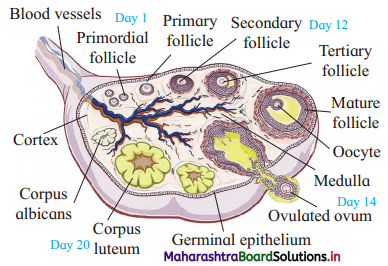
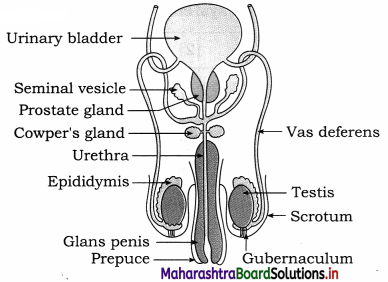
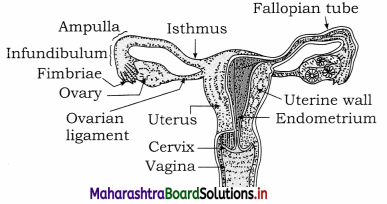
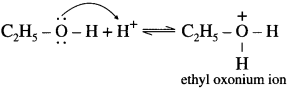
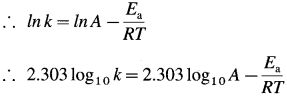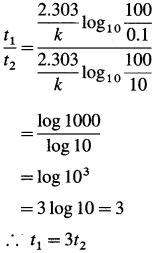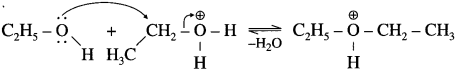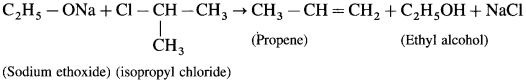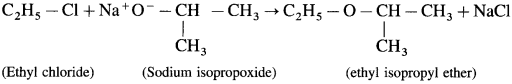Balbharti Yuvakbharati English 12th Digest Chapter 1.1 An Astrologer’s Day Notes, Textbook Exercise Important Questions and Answers.
Class 12 English Chapter 1.1 An Astrologer’s Day Question Answer Maharashtra Board
12th Std English Chapter 1.1 Brainstorming Question Answer
Yuvakbharati English Navneet 12th Digest PDF Free Download Maharashtra Board
Question 1.
Discuss with your partner and complete the table:
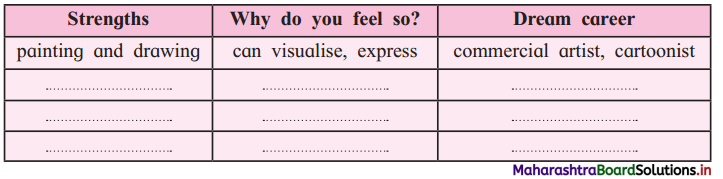
Answer:
| Your Strengths | Why do you feel so? | Your Dream career |
| painting and drawing | can visualise, express | commercial artist, cartoonist |
| ……………………. | ……………………… | …………………………. |
| …………………… | ……………………… | ………………………… |
| ………………….. | ………………………. | ……………………….. |
![]()
Question 2.
The scene in a local market of a village/ town/city is very attractive. People with different occupations sell their wares. Discuss with your partner the variety of activities at the local market.
Answer:
- selling flowers, selling grocery
- selling garments and cloth
- selling imitation jewellery and accessories
- selling snacks and fast food
- selling steel and earthenwares
Question 3.
In a village/town/city it is quite a common sight to see an astrologer sitting by the roadside with his professional equipment. Discuss with your partner and list the requirements for his trade.
Answer:
- parrot, cards, etc.
- turban, beard, dhoti
- dried leaves with writing on them
- cloth with mystic signs to spread his cards
- bead necklace, coins, shells, punchang, etc.
Question 4.
There are certain unreasonable beliefs among people living in our society. Certain common events are linked with superstitions. List such events, discuss the superstitions linked with them and the means of their eradication.
Answer:
Events and superstitions linked with them:
- A cat crossing your path (something bad will happen)
- Walking under a ladder (something unfortunate will happen)
- Wearing black clothes for an auspicious function (will bring bad luck to the hosts)
- Spilling salt (unlucky for the person)
- A black crow cawing outside your window (you will be having guests)
Means of eradication: The only means of eradication is through education. Scientific attitude must be developed in society. Religious heads must counsel and guide their followers. The elders in families must also get rid of old beliefs.
A1.
(i) Given below are some descriptions. Discuss them with your partner and find out one word for each of them.
Question (a)
The scientific study of the universe and the objects in it, including stars, planets, nebulae and galaxies:
Answer:
Astronomy
![]()
Question (b)
The study of the movements of the planets, Sun, Moon, and Stars in the belief that these movements can have an influence on people’s lives:
Answer:
Astrology
Question (c)
A prediction of what will happen in the future:
Answer:
Prophecy
Question (d)
Scientific discipline that studies mental states and processes and behaviour in humans and other animals:
Answer:
Psychology
Question (ii)
In the story we are told that the Town Hall Park was a remarkable place in many ways for an astrologer to build his business. List the exceptional qualities of the place from this extract.
Answer:
The exceptional qualities of the place were:
- A surging crowd
- A variety of trades and occupations, like medicine sellers, sellers of stolen hardware and junk
- magicians
- auctioneers of cheap cloth
- a vociferous vendor of fried groundnuts.
Question (iii)
The astrologer never opened his mouth till the other had spoken for at least ten minutes. Discuss the reasons behind his act.
Answer:
(a) He was good at reading people.
(b) He obtained a lot of information about their lives from their talk.
(c) He could analyse their character and understand their problems.
(d) He could easily frame his statements to their satisfaction.
![]()
A2.
Question (i)
The tactics used by the astrologer to earn his wages are:
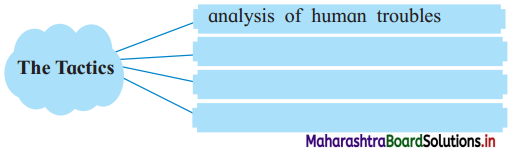
Answer:

Question (ii)
An astrologer’s appearance helps to create an impression on his clients. Complete the following:
(The answer is given directly and underlined.)
Answer:
- The turban on his head
- The sacred ash and vermilion on his forehead
- dark whiskers covering the face
- a sparkle in his eye accompanied by an abnormal gleam
1. Read the following sentences and choose the correct one:
Question (a)
The astrologer says that if Nayak does not leave his village again, he would –
(1) return the money
(2) face danger
(3) go back home and stop looking for the man who tried to kill him
(4) not find the killer.
Answer:
(2) face danger
Question (b)
According to the narrator, the astrologer’s success in his profession is primarily due to –
(1) luck
(2) the bargains he drives
(3) his appearance
(4) his understanding of people.
Answer:
(4) his understanding of people
![]()
Question (c)
The story suggests that the astrologer’s comments and observations pleased people by –
(1) promising them success and good fortune
(2) proving, as time passes, to have been true
(3) flattering them or supporting their own views
(4) helping them to learn to solve their own problems.
Answer:
(3) flattering them or supporting their own views
Question (d)
Guru Nayak the astrologer because he wants to –
(1) understand the past
(2) find out who the astrologer is
(3) make some money through a bet
(4) get the answer to a specific question.
Answer:
(4) get the answer to a specific question.
Question (e)
Guru Nayak is looking for the man who tried to kill him –
(1) to take revenge
(2) to get an apology
(3) to demand an explanation
(4) to prove that the man was unsuccessful.
Answer:
(1) to take revenge
Question (f)
The astrologer’s remarks make Guru Nayak feel all of the following except –
(1) relieved
(2) suspicious
(3) impressed
(4) disappointed.
Answer:
(2) suspicious
![]()
Question (g)
Reactions of the astrologer’s wife to his news suggest that she –
(1) was unaware of his past
(2) has been worried about his safety
(3) has known him since he was young
(4) is concerned about her future with him.
Answer:
(1) was unaware of his past
Question (iv)
Read the following sentences and find out the True and False sentences. Correct the False sentences:
(a) The astrologer gave a correct prediction to the client about his past that he was stabbed, thrown into a well and left for dead
(b) When the astrologer came to know that the man whom he killed is alive he felt that he was relieved of his guilt.
(c) The astrologer tried to back out of the deal and talked about the client’s past.
(d) The astrologer rescued himself from Guru Nayak’s revenge.
(e) The moral of the story is that we must be responsible about what we have done and should not run away from our mistakes.
Answer:
(a) True.
(b) True: When the astrologer came to know that the man whom he killed is alive he felt that he was relieved of his guilt.
(c) False
Corrected sentence. The astrologer struck a bargain with the client and then talked about the client’s past.
(d) True.
(e) False: The moral of the story is that we must be responsible about what we have done and should not run away from our mistakes.
Corrected sentence: The moral is that we should never believe in superstitions.
![]()
Question (v)
The astrologer had changed his appearance and his persona when he arrived in the city. Give specific reasons for this.
Answer:
The astrologer thought that he had killed a man after a quarrel. He was afraid that he would be arrested and jailed for this crime. Hence, to avoid detection he changed his appearance and his persona when he arrived in the city.
Question (vi)
‘The darkness load that was inside the astrologer has disappeared’. Through this sentence, explain the significance of the title ‘An Astrologer’s Day’.
OR
(vii) The astrologer feels relieved that Guru is not dead as it relieves a great burden from him. Critically justify the statement and explain it.
Answer:
The astrologer thought that he had killed a man after a quarrel. Hence he had run away from his village, changed his appearance and his persona when he arrived in the city, and become an astrologer. However, he still felt guilty for what he had done. When he came to know that the man he thought he had killed was actually alive, the dark load inside him disappeared, and it made his day, i.e. he felt relieved and happy. This is the significance of the title ‘An Astrologer’s Day’.
Question (viii)
The astrologer wins/gets the sympathy/ criticism of the reader in the end. Express your opinion with the support of the main story.
Answer:
I think I sympathize with the astrologer. He did not try to intentionally kill Guru Nayak; it had happened in the heat of the moment. Of course, he should not have tried to run away but should have accepted responsibility for his crime. However, he is genuinely sorry for what had happened.
His words ‘a great load is gone from me today. I thought I had the blood of a man on my hands all these years’ indicates this. Hence, I sympathize with him and am happy that he can now live in peace.
![]()
Question (ix)
Suggest some steps to eradicate superstitions and other ill practices from our society.
Answer:
To eradicate superstitions and other ill practices from our society the first and most important step is education. Schools and colleges must help their students to develop a scientific attitude and think logically and rationally.
Secondly, as people in India tend to listen to their religious heads, all religious heads should send out clear messages to their followers about the eradication of superstitions. And lastly, the older generation should change their opinions and ideas and get rid of silly superstitious beliefs.
Question (x)
In the story, the astrologer has great listening power. Listening helps in developing good relations with people. Express your opinion.
Answer:
Yes, listening helps in developing good relations with people. When we listen, we indicate to the speaker that we care about him/her and are interested in his/her problems/joys. We show that we are ready to help him/her if necessary. We share his/ her ideas. We also realize how we can deal with people successfully by listening to their views.
(A3)
Question (i)
In the story, the astrologer, Guru Nayak and astrologer’s wife reveal their qualities through words and actions. Pick out from the box the words that describe them and write in the appropriate columns:
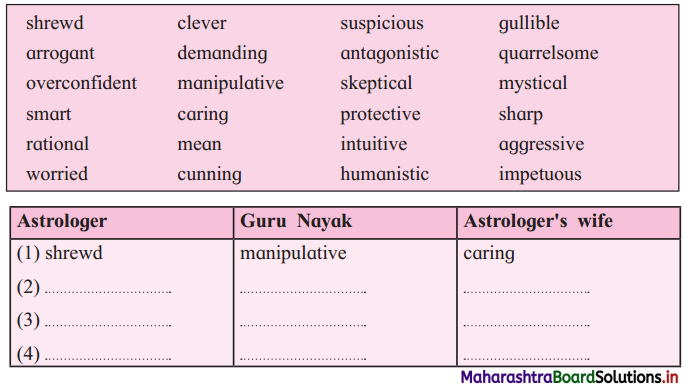
Answer:
| Astrologer | Guru Nayak | Astrologer’s wife |
| shrewd | manipulative | caring |
| clever | gullible | suspicious |
| smart | quarrelsome | protective |
| sharp | arrogant | worried |
| intuitive | aggressive | humanistic |
| mystical | demanding | rational |
| cunning | antagonistic | |
| mean | sceptical | |
| over | impetuous | |
| confident |
![]()
Question 1.
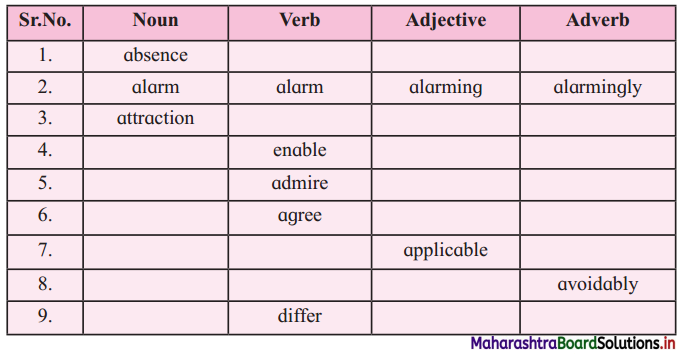
Match the suffixes with the words and make words:
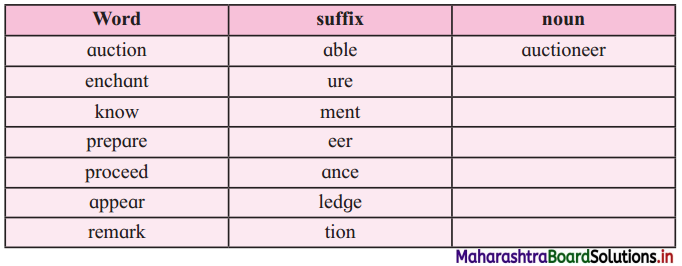
Answer:
| Word | Suffix | Noun |
| auction | able | auctioneer |
| enchantment | enchantment | enchantment |
| know | ment | knowledge |
| prepare | ure | preparation |
| proceed | tion | procedure |
| appear (this word is not in the lesson) | ment | appearance |
| remark | ure | remarkable |
Question (iii)
‘An Astrologer’s Day’ has ironic elements where the astrologer pretends to have ‘supernatural knowledge’ that coincidently turns out to be the truth. Find out an example of irony from the extract and write it down:
Find out the examples of irony from the extract and write them down.
Answer:
His eyes sparkled with a sharp abnormal gleam which was really an outcome of a continual searching look for customers, but which his simple clients took to be a prophetic light and felt comforted.
1. He knew no more of what was going to happen to others than he knew what was going to happen to himself the next minute.
2. He was as much a stranger to the stars as were his innocent customers.
3. He said things which pleased and astonished everyone : that was more a matter of study, practice, and shrewd guesswork.
![]()
Question (iv)
Find the examples of code-mixing from the extract and write them down.
Answer:
1. ‘cowrie shells’
2. turban
Question (v)
There are some phrases where the word ‘crown’ is used with different shades of meaning. Use the following phrases to complete the sentences meaningfully. One is done for you.
Crowning achievement, to crown the effect, crown of thorns, crowning glory, to crown it all
Answer:
e.g. To crown the effect, he wound a saffron- coloured turban around his head.
(a) The works of Shakespeare are the crowning glory of English drama.
(b) Amitabh has given us awesome movies throughout five decades. But his crowning achievement is his performance in the movie ‘Black’.
(c) In her pursuit of success, Radha has distanced herself from her family. Her fame has become a real crown of thorns.
(d) They threw a wonderful party for me with costumes, games and to crown it all my favourite kind of ice cream.
(e) Medical science has great inventions, but organ transplantation is definitely a crowning achievement for human beings.
(A4)
Question (i)
Use the word given in the brackets and rewrite the sentence:
(a) The power of his eyes was considerably enhanced. (enhancement)
(b) He had a working analysis of mankind’s troubles, (worked)
(c) He knew what was going to happen to himself the next minute. (happening)
(d) If you find my answers satisfactory, will you give me five rupees? (satisfaction)
(e) He shook his head regretfully. (regret)
(f) It was a bewildering crisscross of light rays, (bewildered)
(g) “I should have been dead if some passer-by had not chanced to peep into the well,” exclaimed the other, overwhelmed by enthusiasm. (enthusiastically)
(h) You tried to kill him. (killing)
(i) I will prepare some nice stuff for her. (preparation)
(j) The other groaned on hearing it. (heard)
Answer:
(a) There was considerable enhancement in the power of his eyes.
(b) He had worked out an analysis of mankind’s troubles.
(c) He knew what could be happening to himself the next minute.
(d) If my answers give you satisfaction, will you give me five rupees?
(e) He shook his head with regret.
(f) He was bewildered by the crisscross of light rays.
(g) “I should have been dead if some passer-by had not chanced to peep into the well,” exclaimed the other enthusiastically.
(h) You tried killing him.
(i) I will make a preparation of some nice stuff for her.
(j) The other groaned when he heard it.
![]()
(A5)
Question (i)
Prepare a speech on Science and Superstitions.
Answer:
Science and Superstitions
Respected teacher and my dear friends,
I wish you all a very good morning. Today we are celebrating Science day in our school, and on this occasion I, Rohan Kamte, would like to say a few words about Science and Superstitions.
Science and Superstitions are two opposite ends of a pole. Those who have the scientific attitude and believe in science cannot possibly believe in superstitions. After all, what exactly are superstitions? They are only some tales made up by people for some reason or the other. Let me give you an example. Many years ago, in a house in a village, they were having an auspicious function. A lot of food was being cooked.
A cat and her kitten were moving about here and there in the kitchen. Afraid that the cat would be trampled upon or may fall into one of the open fires, the mistress of the house ordered the servant to put the cat and its kitten under a basket, and to do so every time there was a function in the house. This became a ‘superstition’ and in some houses, people actually brought a cat into the house and put it under a basket whenever they had a function!
This is what superstitions are all about. The superstition of bad luck if you walk under a ladder too has its reasons. The ladder could fold up and injure a person walking beneath it, or something could fall on the person’s head.
So friends, I request you: In this age of Science, do not believe in silly superstitions. Keep your minds open. Be rational and logical. Analyse things. Believe something only if it has the backing of Science. Thank you.
(ii) Read the following proverbs. Share you views and expand the ideas.
Question (a)
Actions speak louder than words.
Answer:
Actions speak louder than words
Today a lot of importance is being given to the way we speak and what we speak. But we have to remember that ultimately it is not words but actions that are important. Mahatma Gandhi, the Father of our Nation, did not give any grand speeches. However, by his actions he saw that India gained her freedom. Our soldiers do not give long lectures on patriotism they merely act to defend the country. What would have happened if they had only spoken but not acted?
This very well-known proverb is very apt when it comes to parent-child interaction. It has been seen that children observe the actions of their parents and imitate them not their words. In the animal kingdom too, the actions of the parent are of paramount importance. During elections, politicians make loud speeches but later on do not work. It is because of this behaviour that they lose the trust of the people. Thus, we must act with responsibility, always remembering that people observe our actions and are not swayed by our words.
![]()
Question (b)
The face is the index of the mind.
Answer:
[Points: facial expressions and eyes indicate one’s thoughts – this is. non-verbal communication – that is why we smile when happy and frown when sad – however, smart people can hide their feelings so that face does not show them – so one has to be careful while reading faces]
Question (c)
Speech is silver and silence is golden.
Answer:
[Points: we speak – we give others information or reveal our thoughts – others speak, we get information – sometimes we speak hastily and hurt others – create problems – remain silent and think – can find solutions – many leaders speak hastily – create international problems – better to be silent and let one’s actions speak]
Question (d)
Argument is the worst kind of communication.
Answer:
[Points: arguments – people get angry – angry words and raised voices – hurt people – confusion – relationships spoilt – instead talk softly and allow others to talk – accept that others can think in a different way – ‘a man convinced against his will is of the same opinion still’]
Question (e)
Attitudes are the real figures of speech.
Answer:
[Points: quote by Edwin H Friedman – in communication, more than the verbal message, the non-verbal message important – your attitude and behaviour have more impact than your words – for example, if you say ‘sorry’ in a harsh tone without any apology on your face – the word has no meaning – hence body language and attitude are very important)
Question (f)
The wise man has long ears and a short tongue
Answer:
[Points: better to listen than to speak – wise people listen more and speak only when they have something important to say – speech is silver and silerwe is golden – in any situation it is better to remain silent and evaluate situation – empty vessels make the most noise]
![]()
(A6)
Question (i)
Bill Naughton has written a collection of wonderful stories which you can read in his book ‘The Goal Keeper’s Revenge and Other Stories’. Read all the stories and discuss their themes with your partner.
Question (ii)
Read R.K. Narayan’s humorous collections of short stories and novels. Here are some titles you can read.
(a) ‘Under The Banyan Tree’
(b) ‘The Doctor’s Word’
(c) ‘LawleyRoad’
(d) ‘A Horse and Two Goats’
(e) ‘Gateman’s Gift’
(A7)
Question 1.
Surf the internet and find out the career opportunities in Astronomy.
Yuvakbharati English 12th Digest Chapter 1.1 An Astrologer’s Day Additional Important Questions and Answers
Read the extract and complete the activities given below:
Global Understanding:
Question 1.
List the fancy names the vendor of fried groundnuts gave his wares.
Answer:
The fancy names the vendor of fried groundnuts gave his wares are:
- ‘Bombay Ice Cream’
- ‘Delhi Almond’
- ‘Raja’s Delicacy’, etc.
Question 2.
Complete the following:
(The answer is given directly and underlined.)
Answer:
If the astrologer had stayed in the village, he would have carried on the work of his forefathers-namely, tilling the land, living, marrying and growing old in his cornfield and ancestral home.
![]()
Question 3.
The Town Hall Park was a remarkable place in many ways for an astrologer to build his business. List the exceptional qualities of the place from the extract.
Answer:
The exceptional qualities of the place were:
- lack of municipal lighting
- flare from the groundnut heap
- hissing gaslights, some with naked flares, and cycle lamps
- bewildering criss-cross of light rays and moving shadows
Question 4.
Complete the following:
(The answers are given directly and underlined.)
The signal for the astrologer to leave was when the nuts vendor blew out his flare and rose to go home.
The astrologer spoke only when his client had spoken for at least ten minutes.
Question 5.
Rearrange the following sentences in the order of their occurrence in the extract:
- “I will speak to you tomorrow.”
- “Oh, stop that,” the other said.
- “There is a woman ”
- “Or will you give me eight annas?”
Answer:
- “Oh, stop that,” the other said.
- “Or will you give me eight annas?”
- “I will speak to you tomorrow.”
- “There is a woman ………..”
Complex Factual:
Question 1.
Complete the following:
(The answer is given directly and underlined.) The tactics used by the astrologer to earn his wages are:
Answer:

Question 2.
Describe how the astrologer had left the village.
Answer:
The astrologer had left the village without any previous thought or plan. He had left home without telling anyone. He did not rest till he left behind his village a couple of miles.
![]()
Question 3.
The astrologer could understand the problem in five minutes. Give reasons from the extract.
Answer:
The astrologer had a working analysis of mankind’s troubles like marriage, money and the tangle of human ties. Long practice had sharpened the way he perceived things, and thus he could understand the problem in five minutes.
Question 4.
Complete the following :
(The answer is given directly and underlined.)
Answer:
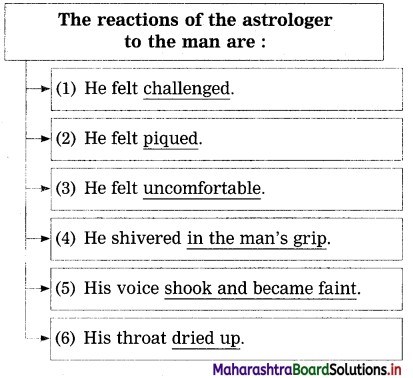
Question 5.
Complete the web:
(The answers are given directly and underlined.)
Answer:

Question 6.
Complete the following with what had happened to Guru Nayak’s enemy, according to the astrologer. According to the astrologer ………….. .
Answer:
According to the astrologer, Guru Nayak’s enemy had died. He had been crushed by a lorry.
![]()
Question 7.
Describe the load on the astrologer’s mind.
Answer:
The astrologer thought that he had killed a man after a quarrel. He felt intensely guilty about this, and had run away from his village. This feeling of guilt was the load on his mind.
Question 8.
Was the astrologer’s wife happy with his day’s earnings? What did she plan to do with it?
Answer:
Yes, the astrologer’s wife was overjoyed with his day’s earnings. She planned to buy some jaggery and coconut and make some sweets for their daughter.
Inference/Interpretation/Analysis:
Question 1.
The presence of the groundnut vendor is beneficial to the astrologer. Justify.
Answer:
The vendor of fried groundnuts gave his wares fancy names like ‘Bombay Ice Cream’, ‘Delhi Almond’, ‘Raja’s Delicacy’ and so on. People were amused and attracted by this and flocked to him to buy groundnuts. As the astrologer was seated right next to him, the groundnut vendor’s customers dallied near the astrologer and were probably tempted to consult him.
Question 2.
Pick out the lines that tell you that the astrologer did not have any real knowledge of astrology.
Answer:
- He had not in the least intended to be an astrologer when he began life.
- He knew no more of what was going to happen to others than he knew what was going to happen to himself the next minute.
- He was as much a stranger to the stars as were his innocent customers.
- It was a bewildering crisscross of light rays and moving shadows. This suited the astrologer very well.
Question 3.
The astrologer could tell the person/client about his life. Describe the method he used.
Answer:
The astrologer would listen to his client talk for about ten minutes. He would thus get all the information about his life from him, and then cleverly pose questions which made it appear that he actually knew about the person’s life.
![]()
Question 4.
‘Our friend felt piqued.’ Name the friend and give reasons for him feeling ‘piqued’.
Answer:
‘Our friend’ is the astrologer. He felt piqued because the man cut short his words rudely and told him to tell him something worthwhile. The astrologer was used to people listening eagerly and respectfully to whatever he had to say, and the behaviour of the man showed that he did not value the usual smooth talk. That is why the astrologer felt piqued.
Question 5.
Complete the following:
(The answers are given directly and underlined.)
Answer:
1. The man was left for dead because he had been pushed into a well in a field. Nobody normally looked into the well, and he would have died had there not been a passer-by who chanced to peep into the well.
2. The man looked gratified because his enemy had met his death by being crushed under a lorry. Guru Nayak felt that the man deserved such a terrible fate for what he had done to him.
Personal Response:
Question 1.
Do you like to hear predictions about your future? Give reasons.
Answer:
No, I do not like to hear predictions about my future. I do not believe that any person can foretell what is going to happen in someone’s life. Astrology is just a way of making money from gullible people. I believe that one must work hard and be a good human being if one wants to be successful in life.
Question 2.
Do you think that astrology is an art and can be studied? Discuss.
Answer:
Yes, astrology is an art. There are various methods of predicting the future, like palm-reading, reading the pulse, reading the horoscope, etc. These methods can be studied, or the knowledge can be inherited from one’s ancestors. However, the astrologer must have intuition and talent for this art.
![]()
Question 3.
Explain with examples your reactions when someone challenges you.
Answer:
If the challenge is worthwhile, I take it up. For example, my friend Rohan challenged me to a bicycle race to the top of a nearby hill. I took it up as it was interesting, and I knew I could do it.
However, when my friend Soham challenged me to jump from the first floor of our building, I refused the challenge, as I knew it was dangerous and I was likely to break some bones. Though Soham scoffed at me, and said that he had already done it, I did not let his ridicule bother me.
Language Study:
Question 1.
The power of his eyes was considerably enhanced by their position.
(Rewrite beginning ‘The position …………’)
Answer:
The position of his eyes considerably enhanced their power.
Question 2.
This colour scheme never failed.
(Rewrite as an affirmative sentence.)
Answer:
This colour scheme was always successful.
![]()
Question 3.
He had left his village without any previous thought or plan. (Rewrite using neither … nor …’)
Answer:
He had left his village with neither any previous thought nor plan.
Question 4.
One or two had hissing gaslights. (Identify the part of speech of the underlined word.)
Answer:
hissing – adjective (present participle used as an adjective)
Question 5.
He never opened his mouth till the other had spoken for at least ten minutes.
(Rewrite using ‘only’.)
Answer:
He opened his mouth only after the other had spoken for at least ten minutes.
Question 6.
He looked up and saw a man standing before him. (Rewrite as a simple sentence.)
Answer:
Looking up, he saw a man standing before him.
Question 7.
If I prove you are bluffing, you must return that anna to me with interest. (Pick out the clauses and state their type.)
Answer:
you must return that anna to me with interest-main clause
If I prove you are bluffing-adverb clause of condition
Question 8.
“Tell me something worthwhile.” (Identify the type of sentence.)
Answer:
Imperative sentence.
![]()
Question 9.
Never travel southward again, and you will live to be a hundred. (Rewrite using ‘only if)
Answer:
You will live to be a hundred only if you never travel southward again.
Question 10.
He flung the coins at her and said “Count them. One man gave all that.” (Rewrite in reported speech.)
Answer:
He flung the coins at her and instructed her to count them. He added that one man had given all of it.
Question 11.
I will prepare some nice stuff for her. (Rewrite using the past perfect tense of the verb.)
Answer:
I had prepared some nice stuff for her.
Vocabulary:
Question 1.
Match the suffixes with the words and make words:
Answer:
| Word | Suffix | Noun |
| innocent | able | innocence |
| reason | ledge | reasonable |
Question 2.
Pick out two words from the extract that indicate sound.
Answer:
crackled, hissing
Question 3.
Guess the meaning of ‘pies’
Answer:
pies – is the plural form of pie which is a former bronze coin of India, the 12th part of an anna.
![]()
Question 4.
Find an example of code mixing from the extract and write it down.
Answer:
pies
Question 5.
Find out the examples of irony from the extract and write them down.
Answer:
1. When he told the person before him, gazing at his palm, “In many ways you are not getting the fullest results for your efforts,” nine out of ten were disposed to agree with him.
2. “Most of your troubles are due to your nature. How can you be otherwise with Saturn where he is? You have an impetuous nature and a rough exterior.” This endeared him to their hearts immediately, for even the mildest of us loves to think that he has a forbidding exterior.
Question 6.
Guess the meaning of the words:
- tilting
- bluffing
- glimpse
Answer:
- tilting – to move into a sloping position.
- bluffing – deceiving, lying
- glimpse – to see someone or something for a very short time
Question 7.
Find examples of code mixing from the extract and write them down.
Answer:
- anna
- rupee
- cheroot
- jutka
Question 8.
Guess the meaning of the words:
- passer-by
- peep
- overwhelmed
- groaned
Answer:
- passer-by – a person who happens to be going past something or someone, especially on foot.
- peep – to peer into something cautiously
- overwhelmed – overcome
- groaned – made a low sound of distress.
![]()
Question 9.
Find examples of code mixing from the extract and write them down.
Answer:
1. annas
2. pyol
Question 10.
Find from the extract the antonyms of the following words:
- light
- noise
- few
- dead
Answer:
- light × darkness
- noise × silence
- few × many
- dead × alive
Non-Textual Grammar:
1. Do as directed:
Question 1.
A stone struck the man on the head.
(Rewrite using the passive voice.)
Answer:
The man was struck on the head by a stone.
![]()
Question 2.
You will not recover. Refrain from smoking.
(Rewrite using ‘unless’.)
Answer:
You will not recover unless you refrain from smoking.
Question 3.
He is certainly taller than his brother.
(Rewrite in the positive degree.)
Answer:
His brother is certainly not as tall as he is.
Spot the error in the following sentences:
Question 1.
His mouth watered when he saw a bouquet of grapes.
Answer:
His mouth watered when he saw a bunch of grapes.
![]()
Question 2.
They left their luggages at the railway station.
Answer:
They left their luggage at the railway station.
12th Std English Questions And Answers:
- An Astrologer’s Day Class 12 Questions And Answers
- On Saying “Please” Class 12 Questions And Answers
- The Cop and the Anthem Class 12 Questions And Answers
- Big Data-Big Insights Class 12 Questions And Answers
- The New Dress Class 12 Questions And Answers
- Into the Wild Class 12 Questions And Answers
- Why We Travel Class 12 Questions And Answers
- Chapter 1.8 Voyaging Towards Excellence Class 12 Questions And Answers











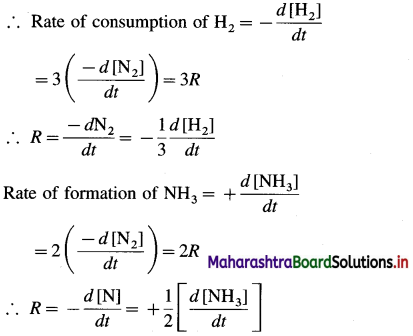
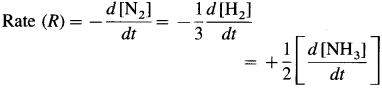

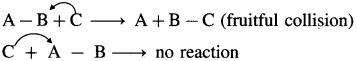
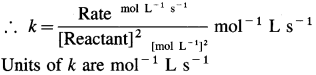
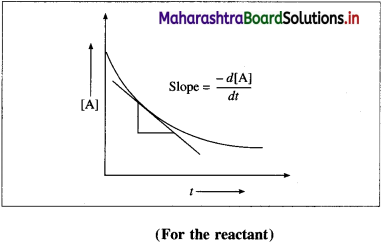
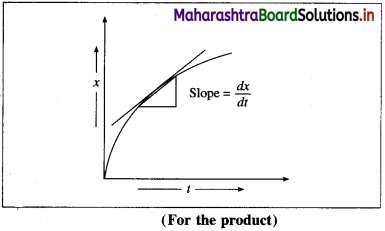
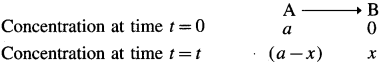
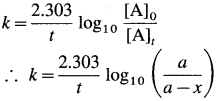

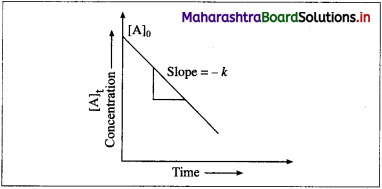

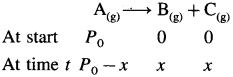
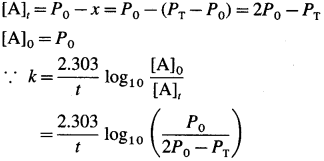
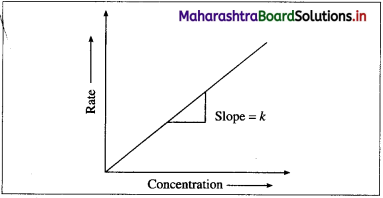

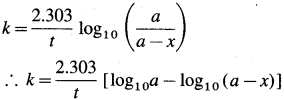


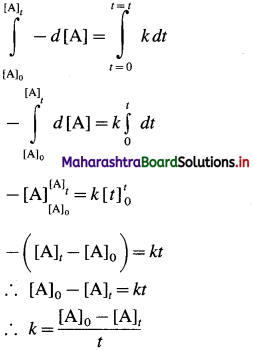
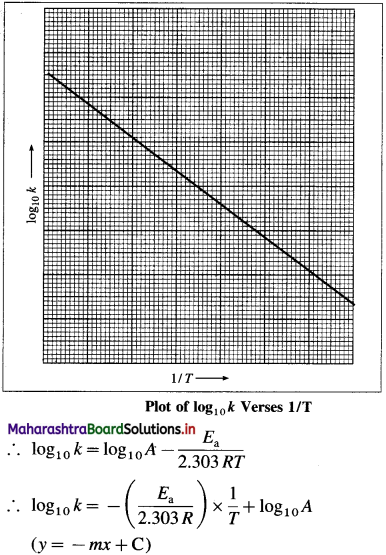
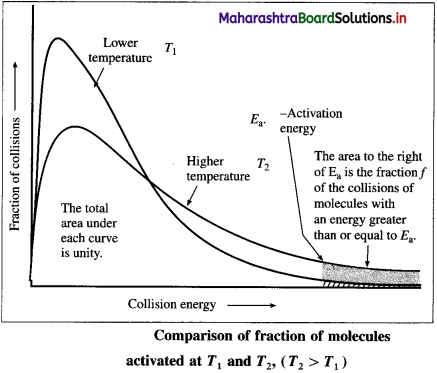
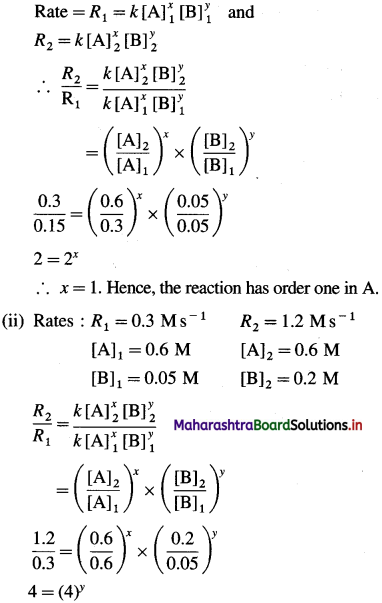
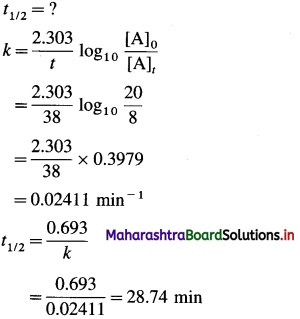
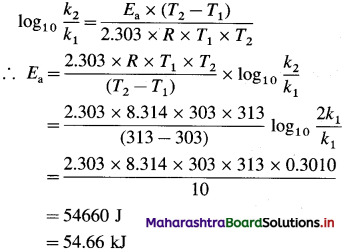
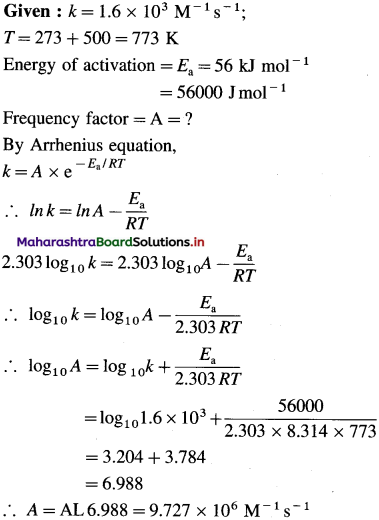
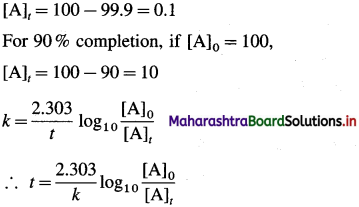
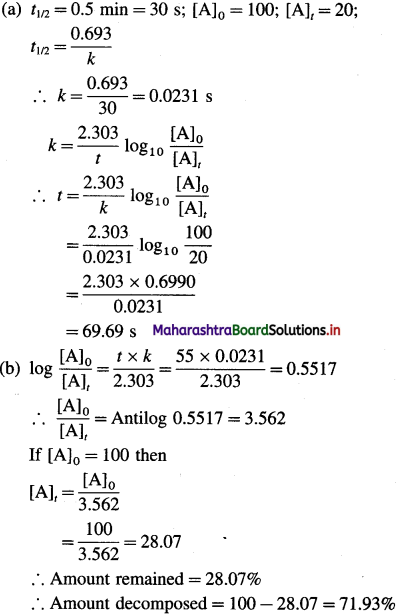
 where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.
where [A0] and [A]t are the respective initial and final concentrations of the reactant after time t.