Balbharti Maharashtra State Board Class 12 Economics Solutions Chapter 10 Foreign Trade of India Textbook Exercise Questions and Answers.
Std 12 Economics Chapter 10 Question Answer Foreign Trade of India Maharashtra Board
Class 12 Economics Chapter 10 Foreign Trade of India Question Answer Maharashtra Board
Economics Class 12 Chapter 10 Question Answer Maharashtra Board
1. Choose the correct option:
Question 1.
Types of foreign trade
a) Import trade
b) Export trade
c) Entrepot trade
d) Internal trade
Options:
1) a and b
2) a, b and c
3) a, b, c and d
4) None of these
Answer:
2) a, b and c
![]()
Question 2.
Export trends of India’s foreign trade includes
a) Engineering goods
b) Gems and Jewellery
c) Textiles and ready-made garments
d) Gold
Options:
1) a and c
2) a, b and c
3) b, c and d
4) None of these
Answer:
2) a, b and c
Question 3.
Role of foreign trade is
a) To earn foreign exchange
b) To encourage investment
c) Lead to division of labour
d) Bring change in composition of exports
Options:
1) a and b
2) a, b and c
3) b and d
4) None of these
Answer:
2) a, b and c
2. Identify and explain the concepts from the given illustrations:
Question 1.
India purchased petroleum from Iran.
Answer:
Concept: Import trade
Explanation: Import trade means purchase of goods and services by one country from another country.
Question 2.
Maharashtra purchased wheat from Punjab.
Answer:
Concept: Internal/Home/Domestic trade Explanation : Internal trade is also known as home trade or domestic trade. This trade is within the country. It is between two or more states of the country.
Question 3.
England imported cotton from India, made readymade garments from it and sold them to Malaysia.
Answer:
Concept: Entrepot trade
Explanation : It means purchase of goods and services from one country and selling the same to another country.
Question 4.
Japan sells smart phones to Myanmar.
Answer:
Concept: Export trade
Explanation : It means sell of goods and services by one country to another country.
3. Distinguish between the following:
Question 1.
Internal trade and International trade.
Answer:
| Internal / Domestic / Home trade | External / Foreign / International trade |
| (a) It means exchange of goods and services within the country. | (a) It means exchange of goods and services between two or more countries. |
| (b) The goods and services are produced and sold within the country. | (b) The goods and services are produced in one country and sold in other country. |
| (c) E.g. Kashmir apples sold in Maharashtra. | (c) E.g. Kashmir apples sold in Dubai. |
Question 2.
Trends in imports and Trends in exports of foreign trade.
Answer:
| Trends in imports | Trends in exports |
| (a) It means year wise numerical changes in imports of a country. | (a) It means year wise numerical changes in exports of a country. |
| (b) India’s major imported goods are – petroleum, gold, fertilizers, iron and steel, etc. | (b) India’s major exported goods are engineering goods, petroleum and chemical products, gems and jewellery, etc. |
| (c) Petroleum has highest import percent of 22.6 in 2016-17. | (c) Engineering goods has highest export percent 23.7 in 2016-17. |
Question 3.
Balance of payments and Balance of trade.
Answer:
| Balance in payment | Balance in trade |
| (a) It means systematic recording of all international economic transactions of that country during a year. | (a) It means the difference between the value of a country’s exports and imports in a year. |
| (b) It is a broad concept. | (b) It is narrow concept. |
4. Answer the following:
Question 1.
Explain the concept of foreign trade and its types.
Answer:
Foreign trade is the exchange of goods and c services between two or more countries, Foreign trade is the trade across the j boundaries of a country.
There are three important types of foreign trade.
- Import trade : It is a buying of goods and services from other country by home country. Excessive import can have a negative impact on home country. E.g. India buying petroleum from Iraq, Kuwait, etc.
- Export trade : It is selling of goods and services by home country to another country.
Excessive export can have a positive impact on the home country. E.g. India exporting tea and spices to USA, China, etc. - Entrepot trade : It means buying of goods and services from one country and selling them to another country. E.g. England importing cotton from India, making readymade garments from it and selling them to Malaysia.
![]()
Question 2.
Explain any four features of composition of Indias foreign trade.
Answer:
There are many changes in India’s foreign trade from last seven decades (70 years)
- Gross National Income : India’s foreign trade has great significance for its GNP. It increased upto 48.8% in the year 2016-17.
- Change in composition of exports : After independence there was change in the composition of India’s export trade from primary products to manufactured goods.
- Change in composition of imports :
After independence there was change in the composition of India’s import trade from consumer goods to capital goods. - Development of new ports : India’s foreign trade is handled mainly by Mumbai, Calcutta and Chennai ports. India has developed more new ports at Kandla, Cochin, Vishakhapatnam.
- Oceanic trade : Most of India’s foreign trade is by sea. About 68% of India’s trade is by sea.
Question 3.
Explain the trend in India’s imports.
Answer:
India is importing various goods from other countries. Following are the major imported goods of India :
- Petroleum : It has largest share in India’s import. In the year 2016-17, it has 22.6% share in India’s total import.
- Gold: After petroleum, the second most imported item is gold. In the year 2011, ) India’s import of gold was $53.9 billion and in the year 2018-19 it declined upto $32.8 billion.
- Fertilizers: The share of fertilizers in import expenditure declined from 4.1% in 1990-91 to only 1.3% in 2016-17.
- Iron and Steel: In the year 2016-17, the share of iron and steel in India’s total import was 2.1%.
5. State with reasons whether you agree or disagree with the following statements:
Question 1.
During British nile, indigenous handicrafts suffered a severe blow.
Answer:
Yes, I do agree with this statement.
- During the British rule India was exporting raw materials to England and was importing final goods from England.
- Indian handicraft was unable to face competition with imported goods from England.
- An imported goods were cheaper as compared to handicraft goods.
- The demand for machine made cheap commodity had raised in Indian market.
- That’s why Indian handicraft industries suffered during the British rule.
Question 2.
Trade is an engine of growth for an economy.
Answer:
Yes, I agree with this statement.
- Trade permits a more efficient allocation of national resources.
- Foreign trade provide foreign exchange which can be used to import modern machinery and technology from advanced countries.
- Foreign trade encourages producers to produce more goods for export.
- It leads to an increase in total investment in an economy.
- Thus, we can say, trade is an engine to growth for an economy.
Question 3.
Foreign trade leads to division of labour and specialization at world level.
Answer:
Yes, I agree with this statement.
- Some countries have abundant natural resources.
- These countries should export raw material and import finished goods from countries which are advanced in skilled man power
- Under specialisation specific work is given to the workers within a production process.
- Specialisation can increase the productivity of a firm or economy.
- Eg. Incase of car manufacturing company, some workers will design the cars, some workers will work on different section of assembly line, some workers will work on j testing cars, some workers will work on marketing of cars.
![]()
6. Observe the following table and answer the questions geven below it.
Direction of Indias imports
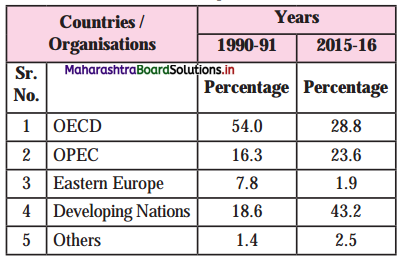
Questions:
Question 1.
Which organisation has the least share in the direction of India’s imports in 2015-16?
Answer:
Eastern Europe has the least share in the direction of India’s import.
Question 2.
Which orgamsation has maximum share in India’s direction of imports in 1990-91?
Answer:
OECD [Organisation for Economic Co-operation and Development has maximum share in India’s direction of imports in 1990-91.
Question 3.
Expand the abbreviations of OECD and OPEC
Answer:
OECD : Organisation for Economic Co-operation and Development.
OPEC : Organisation of Petroleum Exporting Countries.
Question 4.
State your opinion regarding the direction of India’s imports from 1990-91 to 2015-16.
Answer:
In the year 1990-91, OECD (54.0%) and in the year 2015-16, Developing nations (43.2%) has the highest share in the direction of India’s imports. India should encourage industries those are producing import substitute goods,which will help to reduce import from developing nations and help to save foreign exchange.
Question 5.
How much is the percentage of increase in the imports of developing nations in 2015-16 as
compared to 1990-9 1?
Answer:
There is 24.6% increase in the imports of developing nations.
7. Answer in detail :
Question 1.
Explain the meaning and role of foreign trade.
Answer:
Trade means buying and selling of goods and services. Foreign trade means when goods and services are exchanged between two or more countries.
According to Wasserman and Hultman “International trade consists of transaction between residents of different countries”.
Role of foreign trade :
- Brings reputation and helps earn goodwill : Exporting country can earn reputation and goodwill in the international market. Eg. Japan in electronic goods- Panasonic, Canon, Sony, Hitachi. Germany in Automobile – BMW, Audi, Mercedes- Benz, Volkswagen, Porsche. USA in food- McDonalds, KFC, USA in computers – Dell HP, IBM.
- Division of labour and specialisation: It helps to increase the productivity of a firm or economy. Under specialisation specific work is given to the workers within a production process. Eg. Some workers will design cars, some workers will work on assembly lines, some workers will work on testing cars, some workers will work on marketing of cars.
- To earn foreign exchange: Foreign trade is playing very important role in earning foreign exchange. This foreign exchange can be used to import advanced technology and machinery from developed countries.
- Encourages investment : Foreign trade leads to an increase in total investment in an economy. The rise in investment help to produce more goods and services for export.
- Availability of multiple choices : Due to availability of imported goods, it helps to improve standard of living of the people in the country.
- Stability in price level : Foreign trade helps to control the changes in price level by keeping demand and supply position stable,
- Helpful during natural calamities : Foreign trade enables a country to import food grains and medicines from other countries to help the affected people.
- Optimum allocation and utlization of resources : Due to foreign trade those goods are produced which have demand in international market. There is maximum allocation and utlisation of resources to produce more goods and services for export.
- Promotes world peace : Foreign trade is bringing countries closer which leads to better understanding, co-operation and integration.
![]()
Question 2.
Explain the recent trends in India’s exports.
Answer:
Export means selling of goods and services by home country to another country. Excessive export can have a positive impact on the home country.
(i) Engineering Goods : Engineering goods includes transport equipment, automobiles and auto components, machinery and instruments. India’s top export item is engineering goods accounting for 22.5% in India’s total export in 2014-15 and this share has increased upto 25% in the year 2017-18. India is exporting engineering goods to Sri Lanka, UAE and USA.
(ii) Petroleum Products : India’s refining capacity increased significantly since 2001-02 due to which India turned a net exporter of petroleum refinery products. In the year 2013-14 the share of petroleum products in total export was 20.1% and in the year 2016-17 it declined upto 11.7%.
(iii) Chemicals and chemical products:
It includes drugs (Medicines) and pharmaceuticals. This is one sector where India is highly competitive on both quality and pricing factor. India became global hub for pharma production. India is exporting its chemicals and chemical products to USA, China and Germany. The share of this item was 10.4% in 2014-15.
(iv) Gems and Jewellery: Gems and Jewellery plays an important role in earning the foreign exchange for India. In the year 2014¬15 the share of Gems and Jewellery was 13.3% in India’s total export and it declined upto 5.32% in the year 2018-19.
(v) Textiles and readymade garments :
India’s readymade garments have huge demand in the international market. India is exporting textiles to USA, China and Bangladesh. India is exporting readymade garments to USA, UAE and UK. In the year 2014-15 India’s export of textile and garments was 11.3% of total export of India and it has declined upto 6.3% in the year 2016-17.
Intext Questions
Try this : (Text Book Page No. 94)
Name the goods exported to and imported from India to China and Japan in recent years
Answer:
| Goods exported by India | Goods imported by India |
| To China : | From China : |
| raw materials and industrial inputs like organic chemicals, mineral fuels, cotton, ores, plastic materials, etc. | electronic items, machinery, and plastic items. |
| To Japan : | From Japan : |
| fisheries products, wheat, tea, coffee, species and herbs. | mineral fuels, machinery and food items. |
Find out: (Text Book Page No. 95)
Find the recent share of India’s foreign trade in Gross National Income.
Answer:
India’s foreign trade accounts for 48.8% of her Gross National Income.
![]()
Find out: (Text Book Page No. 97)
List the countries coming under OPEC and OECD.
Answer:
The countries coming under OPEC (Organisation of Petroleum Exporting Countries) are :
(a) Algeria, (b) Angola, (c) Congo, (d) Equatorial Guinea, (e) Gabon, (f) Iran, (g) Iraq, (h) Kuwait, (i) Libya, (j) Nigeria, (k) Saudi Arabia (1) United Arab Emirates, 0 Venezuela.
12th Std Economics Questions And Answers:
- Introduction to Micro and Macro Economics Class 12 Economics Questions And Answers
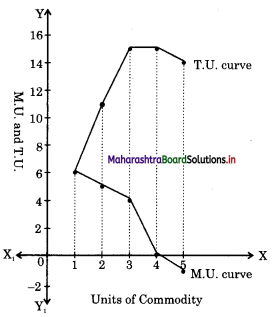
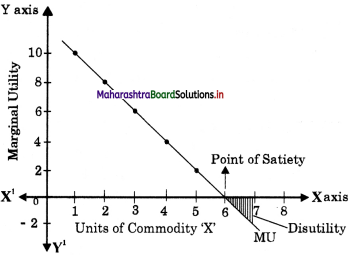
- Utility Analysis Class 12 Economics Questions And Answers
- Demand Analysis Class 12 Economics Questions And Answers
- Elasticity of Demand Class 12 Economics Questions And Answers
- Supply Analysis Class 12 Economics Questions And Answers
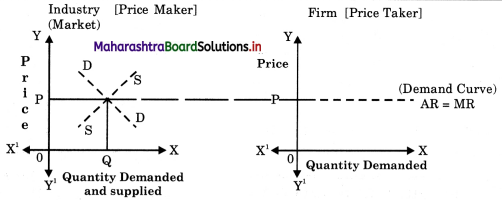
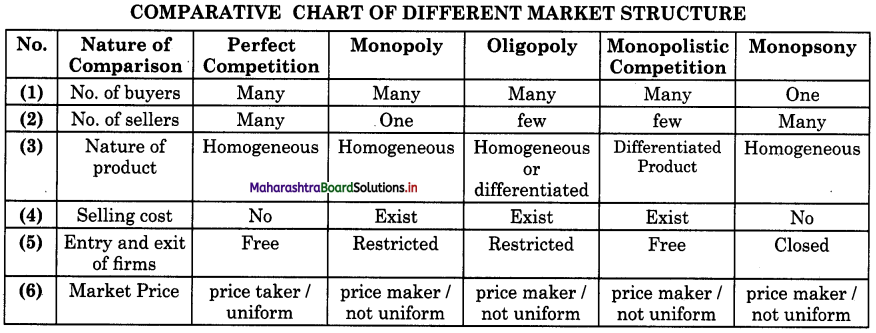
- Forms of Market Class 12 Economics Questions And Answers
- Index Numbers Class 12 Economics Questions And Answers
- National Income Class 12 Economics Questions And Answers
- Public Finance in India Class 12 Economics Questions And Answers
- Money Market and Capital Market in India Class 12 Economics Questions And Answers
- Foreign Trade of India Class 12 Economics Questions And Answers