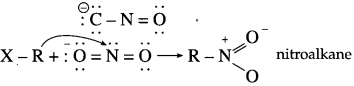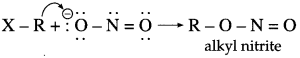Balbharti Maharashtra State Board Marathi Yuvakbharati 12th Digest Chapter 3 आयुष्य… आनंदाचा उत्सव Notes, Textbook Exercise Important Questions and Answers.
Maharashtra State Board 12th Marathi Yuvakbharati Solutions Chapter 3 आयुष्य… आनंदाचा उत्सव
12th Marathi Guide Chapter 3 आयुष्य… आनंदाचा उत्सव Textbook Questions and Answers
कृती
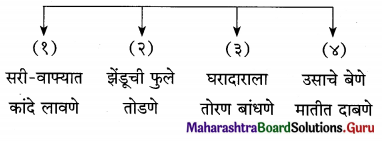
1. अ. कृती करा.
प्रश्न अ.
कृती करा.
उत्तर :
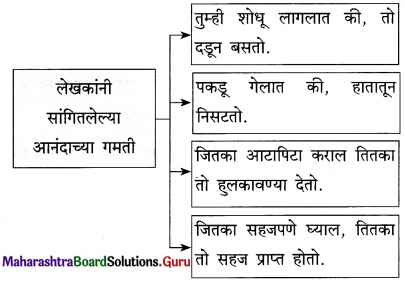
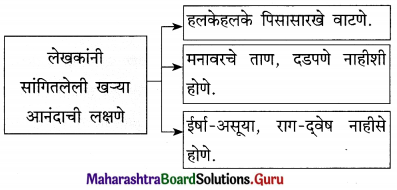
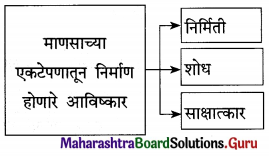
आ. खालील विधाने योग्य की अयोग्य ते लिहा.
प्रश्न 1.
- यश, वैभव ही आनंद अनुभवण्याची निमित्तं आहेत.
- पैशाने आनंद विकत घेता येऊ शकतो.
- शिकण्यातला आनंद तात्पुरता असतो.
- यशामुळे आत्मविश्वास वाढतो.
- ज्यात तुम्हांला खरा आनंद वाटतो, तेच काम करा.
उत्तर :
- योग्य
- अयोग्य
- अयोग्य
- योग्य
- योग्य
![]()
इ. हे केव्हा घडेल ते लिहा.
प्रश्न 1.
- माणसाला आनंद दुसऱ्याला वाटावासा वाटतो, जेव्हा …….
- माणूस दु:खातून बाहेर पडत नाही, जेव्हा …….
- आनंद हा तुमचा स्वभाव होईल, जेव्हा ……..
- एका वेगळ्या विश्वात वावरता येतं, जेव्हा ……
उत्तर :
- माणसाला आनंद दुसऱ्याला वाटावासा वाटतो, जेव्हा त्याच्या मनात आनंद मावेनासा होतो.
- माणूस दु:खातून बाहेर पडत नाही, जेव्हा तो दुःखाला स्वत:च्या मनाबाहेर जाऊ देत नाही.
- आनंद हा तुमचा स्वभाव होईल, जेव्हा आनंदातच राहायची सवय तुम्हांला पडते.
- एका वेगळ्या विश्वात वावरता येते, जेव्हा आपण एखाद्या कलेशी दोस्ती करतो.
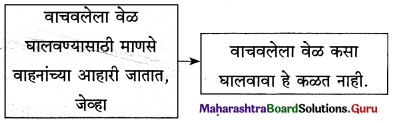
2. अ. खालील शब्दसमूहांचा तुम्हांला समजलेला अर्थ लिहा.
प्रश्न 1.
मनाची कवाडं-
उत्तर :
मनाची कवाडं : मनाची कवाडं म्हणजे मनाची दारे. घराचे दार उघडल्यावर आपण बाहेरच्या जगात प्रवेश करतो. घरातले विश्व चार भिंतीच्या आतले असते. ते संकुचित असते. बाहेरचे जग अफाट असते. दार आपल्याला अफाट जगात नेते. मनाची दारे उघडली, तर म्हणजे मन मोकळे ठेवले, तर आपण व्यापक जगात प्रवेश करतो.
![]()
प्रश्न 2.
आनंदाचा पाऊस-
उत्तर :
आनंदाचा पाऊस : मनात दुःख, चिंता असेल, तर आनंद मनात शिरत नाही. आनंदाचे खुल्या मनाने स्वागत करावे लागते. मन मोकळे ठेवले तर आनंद भरभरून मनात शिरतो. यालाच आनंदाचा पाऊस म्हटले आहे.
आ. खालील चौकटी पूर्ण करा.
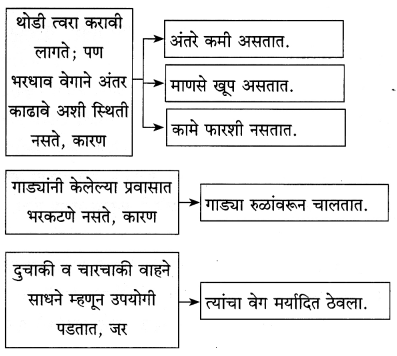
प्रश्न 1.
- आनंदाला आकर्षित करणारा – [ ]
- शरीर आणि मन यांना जोडणारा सेतू – [ ]
- बाहेर दाराशी घुटमळणारा – [ ]
- आनंदाला प्रसवणारा – [ ]
- आनंद अनुभवण्याची निमित्तं – [ ] [ ]
उत्तर :
- आनंदाला आकर्षित करणारा – आनंद
- शरीर आणि मन यांना जोडणारा सेतू – श्वास
- बाहेर दाराशी घुटमळणारा – आनंद
- आनंदाला प्रसवणारा – आनंद
- आनंद अनुभवण्याची निमित्तं – यश वैभव
3. व्याकरण.
अ. खालील वाक्यांचा प्रकार ओळखून लिहा.
प्रश्न 1.
- एवढं मिळवूनही मी आनंदात का नाहीये? …………………….
- ‘गोडधोड’ हे सुद्धा पूर्णब्रह्मच असतं की! …………………….
- आनंदासाठी मन मोकळं असावं लागतं. …………………….
उत्तर :
- प्रश्नार्थी वाक्य
- उद्गारार्थी वाक्य
- विधानार्थी वाक्य.
आ. योग्य पर्याय निवडा व लिहा.
प्रश्न 1.
माणसं स्वत:चा छंद कसा विसरू शकतात? या वाक्याचे विधानार्थी वाक्य
(अ) माणसं स्वत:चा छंद नेहमी विसरतात.
(आ) माणसं स्वत:चा छंद लक्षात ठेवतात.
(इ) माणसं स्वत:चा छंद विसरू शकत नाहीत.
(ई) माणसं स्वत:चा छंद किती लक्षात ठेवतात.
उत्तर :
(इ) माणसं स्वत:चा छंद विसरू शकत नाहीत.
![]()
प्रश्न 2.
हा आनंद सर्वत्र असतो. या वाक्याचे प्रश्नार्थी वाक्य
(अ) हा आनंद कुठे नसतो?
(आ) हा आनंद कुठे असतो?
(इ) हा आनंद सर्वत्र नसतो का?
(ई) हा आनंद सर्वत्र असतो का?
उत्तर :
(अ) हा आनंद कुठे नसतो?
प्रश्न 3.
किती आतून हसतात ती! या वाक्याचे विधानार्थी वाक्य
(अ) ती आतून हसतात.
(आ) ती फार हसतात आतून.
(इ) ती आतून हसत राहतात.
(ई) ती खूप आतून हसतात.
उत्तर :
(ई) ती खूप आतून हसतात.
इ. खालील तक्ता पूर्ण करा.
प्रश्न 1.
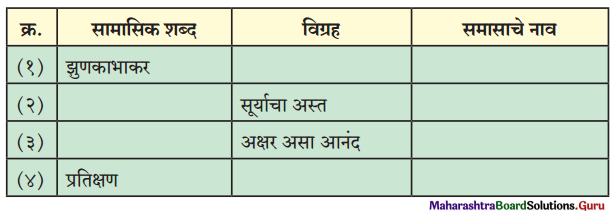
उत्तर :
| सामासिक शब्द | विग्रह | समासाचे नाव |
| झुणका भाकर | झुणका, भाकर वगैरे | समाहार द्वंद्व |
| सूर्यास्त | सूर्याचा अस्त | विभक्ती तत्पुरुष |
| अक्षरानंद | अक्षर असा आनंद | कर्मधारय |
| प्रतिक्षण | प्रत्येक क्षणाला | अव्ययीभाव |
ई. खालील वाक्यांतील प्रयोग ओळखा व लिहा.
प्रश्न 1.
- स्वत:च्या आवडीचे काम निवडा ………..
- लोकांना पेढे वाटणं वेगळं ………..
- कष्टाची भाकर गोड लागते ………..
उत्तर :
- स्वत:च्या आवडीचे काम निवडा. कर्तरी प्रयोग
- लोकांना पेढे वाटणं वेगळं. भावे प्रयोग
- कष्टाची भाकर गोड लागते. कर्तरी प्रयोग
उ. ‘आनंद’ या शब्दासाठी पाठात आलेली विशेषणे शोधा व लिहा.
प्रश्न 1.
‘आनंद’ या शब्दासाठी पाठात आलेली विशेषणे शोधा व लिहा.
…………. ………… ………….. ………… …………
उत्तर :
- खरा (आनंद)
- आत्मिक (आनंद)
- अनोखा (आनंद)
- वेगळा (आनंद)
- टिकाऊ (आनंद).
4. स्वमत.
प्रश्न अ
‘जे काम करायचचं आहे, त्यात आनंद घ्यायला शिकणं हेही शक्य असतं’, या विधानाबाबत तुमचे मत सविस्तर लिहा.
उत्तर :
शिक्षण घेताना आपण आपल्या आवडीचा विषय घेऊ शकतो, हे खरे आहे. काही वेळा आईवडिलांच्या आग्रहाला आपण बळी पडतो किंवा आपले सर्व मित्र जिकडे जातात, ती शाखा आपण निवडतो. कालांतराने आपली आपल्याला चूक उमगते. पण उशीर झालेला असतो. त्यानंतर काहीही करता येत नाही. निराश मनाने आपण शिक्षण घेतो अणि आयुष्यभर तशाच मन:स्थितीत जीवन जगत राहतो. त्यात सुख अजिबात नसते.
शिक्षणानंतर नोकरी-व्यवसाय निवडताना तसाच प्रश्न उद्भवतो. इथे मात्र आपल्याला निवड करण्याची बरीच संधी असते. या वेळी आपण आवडीचे क्षेत्र निवडायला हवे. क्षेत्र आवडीचे असल्यास आपण आनंदाने काम करू शकतो. मग काम कष्टाचे राहत नाही. आपल्या कामातून, कामाच्या कष्टातून आनंद मिळू शकतो.
मात्र इथेही एक अडचण असतेच. पण आवडीच्या विषयातील ज्ञान मिळवलेले असले, तरी नोकरी-व्यवसाय आवडीचाच मिळेल याची खात्री नसते. शिक्षण घेतलेले लाखो विद्यार्थी असतात. पण नोकऱ्या मात्र संख्येने खूप कमी असतात. त्यामुळे आपल्या आवडीची नोकरी आपल्याला मिळेल याची खात्री नसते. उपजीविका तर पार पाडायची असते. त्यामुळे मिळेल ती नोकरी स्वीकारावी लागते. अशा वेळी काय करायचे?
अशा वेळी वाट्याला आलेली नोकरी किंवा व्यवसाय आनंदाने केला पाहिजे. पण आनंदाने करायचा म्हणजे काय करायचे? कसे करायचे? तोपर्यंत आपण जे शिक्षण घेतलेले आहे, त्यातील सर्व ज्ञान, सर्व कौशल्ये पणाला लावली पाहिजेत. मग आपले काम आपल्याला अधिक जवळचे वाटू लागेल. तसेच, एवढे प्रयत्न अपुरे पडले तर आपले काम उत्तमातल्या उत्तम पद्धतीने करण्यासाठी गरज पडली, तर नवीन कौशल्ये शिकून घेतली पाहिजेत. काहीही करून आपले काम सर्वोत्कृष्ट झाले पाहिजे, असा आग्रह हवा. मग आपोआपच आपले काम सुंदर होईल. आपल्याला आनंद मिळेल आणि आपल्या कामाला प्रतिष्ठाही मिळेल.
![]()
प्रश्न आ.
‘सौंदर्य जसं पाहणाऱ्याच्या दृष्टीत असतं, तसा आनंद घेणाऱ्याच्या वृत्तीत असतो’, या विधानाबाबत तुमचे मत स्पष्ट करा.
उत्तर :
एखादी व्यक्ती काहीजणांना सुंदर दिसते. तर अन्य काहीजण ती सुंदर नाहीच, यावर पैज लावायला तयार होतात. हा व्यक्ति – व्यक्तींच्या दृष्टींतला फरक आहे. कोणत्या कारणांनी कोणती व्यक्ती कोणाला आवडेल हे काहीही सांगता येत नाही. त्याप्रमाणे कोणाला कशात आनंद मिळेल, हेही सांगता येत नाही. आनंदाच्या तऱ्हा वेगवेगळ्या असतात. प्रत्येकाचा आनंद वेगळा असतो. पोस्टाची तिकिटे किंवा नाणी गोळा करण्याचा नेहमीचा छंद असलेली माणसे आपल्याला ठाऊक असतात. पण एकाला लोकांकडची जुनी पत्रे गोळा करण्याचा छंद होता.
एकजण आठवड्यातून एकदा आसपासचा एकेक गाव पायी चालून यायचा. एकच सिनेमा एकाच महिन्यात सात-आठ वेळा पाहणारेही सापडतात. सिनेमातले सर्व संवाद त्यांना तोंडपाठ असतात. ते संवाद ते सिनेमाप्रेमी पुन्हा पुन्हा ऐकवतात. यातून त्याला कोणता आनंद मिळत असेल? यावरून एकच दिसते की, प्रत्येकाची आनंदाची ठिकाणे भिन्न असतात. आनंद शोधण्याची वृत्ती भिन्न असते.
व्यक्तिव्यक्तींमधला हा वेगळेपणा आपण लक्षात घेतला, तर समाजातील अनेक भांडणे संपतील; समाजासमोरच्या समस्यासुद्धा सुटतील. प्रत्येक व्यक्तीची प्रकृती भिन्न असते. आवडीनिवडी भिन्न असतात. हे वास्तव आपण ओळखले पाहिजे.
व्यक्तींची ही विविधता ओळखली पाहिजे. या विविधतेची बूज राखली पाहिजे. मग समाजात विविध प्रकारच्या रंगीबेरंगी वस्तू निर्माण होतील. रंगीबेरंगी घटना घडत राहतील. समाजजीवन अनेक रंगांनी बहरून जाईल.
प्रश्न इ.
‘आनंदाचं खुल्या दिलानं स्वागत करावं लागतं’, या विधानाचा तुम्हाला कळलेला अर्थ स्पष्ट करा.
उत्तर :
एखादया दिवशी आपल्याला नको असलेला माणूस भेटतो. “कशाला भेटली ही ब्याद सकाळी सकाळी!” असे आपण मनातल्या मनात म्हणतो. तरीही आपण तोंड भरून हसत स्वागत करतो. आपल्या बोलण्यात, हसण्यात खोटेपणा भरलेला असतो. हे असे बऱ्याच वेळा होते. आपण खोटेपणाने जगतो. भेटलेल्या व्यक्तीमुळे आपल्याला आनंद होतच नाही.
आनंदाचा, सुखाचा अनुभव आपल्याला मिळतच नाही; कारण आपले मन आधीच राग, द्वेष, मत्सराच्या भावनांनी भरलेले. अशा भावनांच्या वातावरणात आनंद निर्माण होऊच शकत नाही. मन ढगाळलेले असले की तेथे स्वच्छ सूर्यप्रकाश येऊच शकत नाही.
आनंदाचा, सुखाचा अनुभव मिळण्यासाठी आपले मन निर्मळ असले पाहिजे. कुत्सितपणा, द्वेष, मत्सर, हेवा असल्या कुभावनांपासून मन मुक्त हवे. जेथे कुभावनांची वस्ती असते, तेथे निर्मळपणा अशक्य असतो. निर्मळपणा असला की मन मोकळे होते. स्वच्छ होते. अशा मनातच आनंदाचा पाऊस पडतो. आपल्याला खरे सुख, खरा आनंद हवा असेल, तर मन स्वच्छ, मोकळे असले पाहिजे; कुभावनांना तिथून हाकलले पाहिजे.
आमच्या शेजारी सिद्धा नावाची बाई राहते. सिद्धाच्या मनात समोरच राहणाऱ्या अमिताविषयी दाट किल्मिषे भरलेली आहेत. अमिताविषयी बोलताना ती सर्व किल्मिषे जळमटांसारखी सिद्धाच्या तोंडून बाहेर पडतात. सिद्धा निर्मळ मनाने अमिताकडे पाहूच शकत नाही. साहजिकच अमिताच्या सहवासाचा सिद्धाचा अनुभव कधीही सुखकारक, आनंददायक नसतो.
ज्या ज्या वेळी अमिताविषयी बोलणे निघते, त्या त्या वेळी सिद्धाचे मन कडवट होते. मनात कुभावनांचे ढग घेऊन वावरण्यामुळे सिद्धाला आनंद, खराखुरा आनंद मिळूच शकत नाही. लेखकांनी ‘आनंदाच खुल्या दिलानं स्वागत करावं लागतं,’ असे म्हटले आहे, ते खरेच आहे.
![]()
प्रश्न ई.
‘प्रत्येक माणसाला आपल्या अस्तित्वाचे भान असणे अत्यंत गरजेचे आहे’, तुमचे मत लिहा.
उत्तर :
प्रत्येकाला आपल्या अस्तित्वाचे भान असणे आवश्यक आहे; हे अगदी खरे आहे. आपण हे भान बाळगत नाही. त्यामुळे आपले नुकसानही होते. आपल्या साध्या साध्या कृतींकडे लक्ष दिले, तरी हा मुद्दा लक्षात येईल. रस्ता ओलांडताना भरधाव येणाऱ्या गाड्यांना आपण लीलया चुकवत चुकवत जातो. खो-खोमध्ये किती चपळाई दाखवतो आपण! आपण सवयीने या हालचाली करतो.
त्यामुळे त्यांतली किमया आपल्या लक्षातच येत नाही. ‘चक दे इंडिया हा चित्रपट पाहताना है खूपदा लक्षात आले आहे. सर्व हालचाली करताना आपण आपल्या शरीराचा उपयोग करतो. ‘हे माझे शरीर आहे आणि या शरीराच्या आधाराने मी जगतो,’ ही भावना सतत जागी असली पाहिजे. मग आपल्या प्रत्येक हालचालीचा आपण बारकाईने विचार करू शकतो. शरीराला प्रशिक्षण देऊ शकतो. अनेकदा आपल्याला नाचण्याची लहर येते. पण पावले नीट पडत नाहीत. आपण मनातल्या मनात खटू होतो. पण शरीराची जाणीव असेल, तर नृत्यातल्या हालचाली शिकून घेता येतात. तिथेच आपली चूक होते.
खरे तर प्रत्येक पाऊल टाकताना आपण आपल्या शरीराचा डौल राखला पाहिजे. कोणाही समोर जातो, तेव्हा हेच लक्षात ठेवले पाहिजे. आपण इतरांसमोर स्वत:ला सादर करीत असतो. ते सादरीकरण सुंदर केले पाहिजे. आपल्याला लाभलेले अस्तित्व प्रत्येक क्षणाला साजरे केले पाहिजे. तर मग आपण जगण्याचा आनंद घेऊ शकतो.
अभिनेते, खेळाडू अनेक कसलेले सादरकर्ते डौलदार का दिसतात? एखादी अभिनेत्री फोटोसाठी उभी राहते, तेव्हा तीच लक्षणीय का दिसते? ही सगळी माणसे आपल्या देहाचे, आपल्या अस्तित्वाचे भान बाळगतात. आपले अस्तित्व देखणे करायचा प्रयत्न करतात. ती स्वत:च्या अस्तित्वाचा आनंद घेतात आणि दुसऱ्यांना देतातही. हेच सुख असते. त्यातच आनंद असतो.
5. अभिव्यक्ती.
प्रश्न अ.
खरा, टिकाऊ आनंद मिळवण्यासाठी करावे लागणारे प्रयत्न तुमच्या शब्दांत लिहा.
उत्तर :
टिकाऊ आनंद मिळवण्यासाठी सर्वप्रथम टाकायचे पाऊल म्हणजे स्वत:च्या शरीरावर प्रेम करणे. आपण स्वत: असे प्रेम करायचेच; पण इतरांनाही तो मार्ग शिकवायचा.
स्वत:च्या शरीरावर प्रेम करायचे म्हणजे काय करायचे? शरीर नीटनेटके, स्वच्छ व प्रसन्न राखायचे. आपल्याला पाहताच कोणालाही आनंद झाला पाहिजे. त्याला प्रसन्न वाटले पाहिजे. त्यासाठी स्वच्छतेच्या सवयी अंगी बाणवल्या पाहिजेत. आहार विचारपूर्वक घ्यायचा, व्यसने करायची नाहीत, दरोज नियमितपणे योगासने किंवा अन्य व्यायाम किंवा रोज तीन-चार किमी चालणे. कामासाठी चालणे यात मोजायचे नाही. काहीही करण्यासाठी नव्हे, तर चालण्यासाठी चालायचे. चालणे हेच काम समजायचे.
मनात ईर्षा, असूया, हेवा, मत्सर, सूड अशा कुभावना बाळगायच्या नाहीत. आपले मन या भावनांपासून दूर ठेवण्यासाठी म्हणजे चांगले होण्यासाठी स्वत: कोणत्या तरी एका क्षेत्रात, एखाद्या कौशल्यात प्रभुत्व मिळवले पाहिजे. स्वतःच्या कर्तबगारीवर विश्वास ठेवायचा. त्यामुळे अन्य कोणाहीबद्दल मनात कुभावना बाळगण्याची इच्छाच होणार नाही.
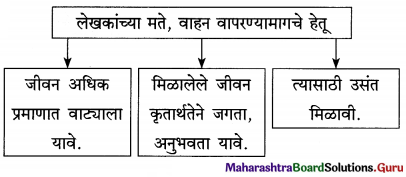
यश, वैभव मिळवण्याचा प्रयत्न करण्यात गैर काहीच नाही. मात्र यश, वैभव या गोष्टी बाह्य असतात. आत्मिक समाधानाशी संबंध नसतो. म्हणून यश, वैभव मिळाल्यावरही मन अशांत, अस्वस्थ होऊ शकते. अशा वेळी आणखी यश, आणखी वैभव यांच्या मागे न लागता आपल्याला नेमके काय हवे आहे. याचा शोध घेतला पाहिजे.
मात्र, एक गोष्ट कायम लक्षात ठेवली पाहिजे. पैशाने खरा, टिकाऊ आनंद कधीही मिळवता येत नाही. आपल्या मनाच्या सोबत राहण्यासाठी आवडेल तेच काम करायला घ्यावे. आवडेल त्या क्षेत्रात नोकरी, व्यवसाय पत्करावा. अर्थात, प्रत्येकाला स्वत:च्या आवडीप्रमाणे नोकरी, व्यवसाय मिळेलच असे नसते. अशा वेळी मिळालेले काम आवडीने केले पाहिजे.
एवढी पथ्ये प्रामाणिकपणे पाळली तर आपण खऱ्या आनंदाच्या जवळ असू.
![]()
प्रश्न आ.
तुमचे जीवन आनंदी होण्यासाठी तुम्ही काय काय कराल, ते लिहा.
उत्तर :
जीवन आनंदी होण्यासाठी आवश्यक असलेल्या अनेक गोष्टी मी करीन. त्यापैकी काही कृती शारीरिक पातळीवरील आहेत. तर काही मानसिक पातळीवरील आहेत.
शारीरिक पातळीवरील कृतींपैकी सर्वांत महत्त्वाची कृती म्हणजे स्वत:च्या शरीराची काळजी घेणे. स्वत:च्या शरीराची काळजी घेण्यासाठी प्रथम स्वत:च्या शरीरावर मनापासून प्रेम केले पाहिजे. स्वतःचे शरीर नीटनेटके, देखणे राखायचे, इतके की कोणालाही भेटल्यावर ती व्यक्ती आनंदित, प्रसन्न झाली पाहिजे. शरीर फक्त बाह्यतः सजवून ते देखणे होणार नाही. ते सतेज, सुदृढ व निरोगी राखले पाहिजे. त्या दृष्टीने मी योगासने किंवा व्यायाम सुरू करीन. नियमित व जीवनसत्त्वयुक्त आहाराचा अवलंब करीन. व्यसनांपासून चार हात दूरच राहीन.
शरीराबरोबरच मनाचे पोषण करण्यासाठी मी कलेचा आश्रय घेईन. मी अत्यंत चिकाटीने गायन, वादन, नर्तन, साहित्य, चित्रपट, नाट्य यांपैकी एका तरी कलेचा जाणतेपणाने आस्वाद घ्यायला शिकेन. शक्यतो एखादी कला आत्मसात करीन. माझी स्वत:ची बौद्धिक, शारीरिक व मानसिक क्षमता लक्षात घेऊन माझे यशाचे लक्ष्य निश्चित करीन आणि त्याचा पाठपुरावा करीन. अर्थात मला हेही ठाऊक आहे की केवळ यशामुळे उच्च पातळीवरचे मानसिक समाधान मिळू शकत नाही. साफल्याचा आनंद भौतिक यशाने पूर्णांशाने मिळत नाही. म्हणून कला क्रीडा-ज्ञान या क्षेत्रांत उच्च प्रतीचे कौशल्य मिळवायचा प्रयत्न करीन.
नोकरी-व्यवसायाच्या बाबतीत आवडीचेच क्षेत्र मिळेल असे सांगता येत नाही. मी माझ्या आवडीचे शिक्षण घेईन. आवडीच्या क्षेत्रात उपजीविकेचे साधन मिळवायचा प्रयत्न करीन. तसे नाही मिळाले, तर मिळालेले काम अत्यंत आवडीने करीन. मी घेतलेल्या शिक्षणातून मिळालेले ज्ञान माझ्या नोकरी-व्यवसायात वापरीन.
मला तर खात्रीने वाटते की माझा हा बेत यशस्वी झाला, तर मला सुखीसमाधानी आयुष्य मिळेल.
![]()
उपक्रम :
प्रस्तुत पाठात आलेल्या इंग्रजी शब्दांची यादी करा. त्यांसाठी वापरले जाणारे मराठी शब्द लिहा.
तोंडी परीक्षा.
अ. खालील वाक्प्रचारांचा अर्थ सांगून वाक्यांत उपयोग करा.
1. आभाळाकडे डोळे लावणे.
2. विसर्ग देणे.
आ. ‘माझ्या जीवनातील आनंदाचे क्षण’ या विषयावर पाच मिनिटांचे भाषण सादर करा.
Marathi Yuvakbharati 12th Digest Chapter 3 आयुष्य… आनंदाचा उत्सव Additional Important Questions and Answers
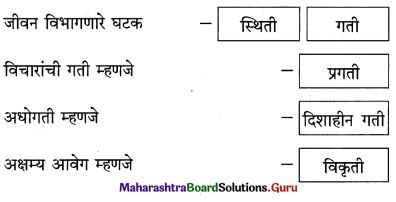
कृती करा.
प्रश्न 1.
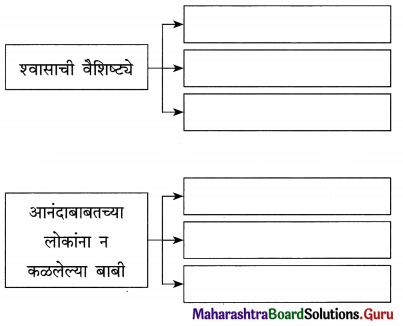
उत्तर :
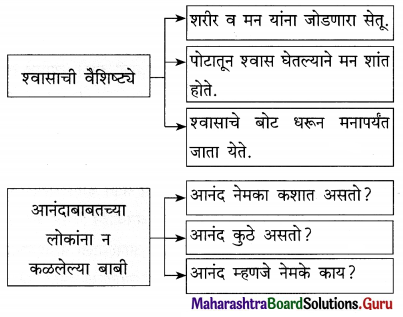
प्रश्न 2.
उत्तर :
प्रश्न 3.
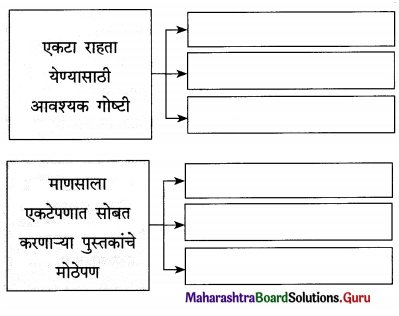
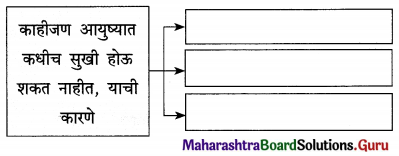
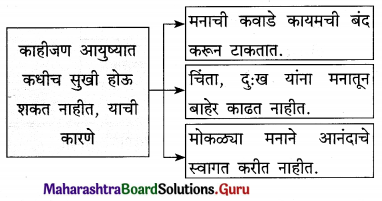
उत्तर :
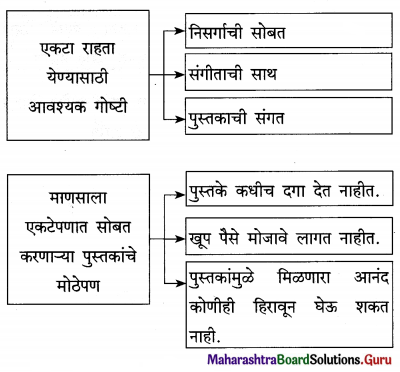
प्रश्न 4.
उत्तर :
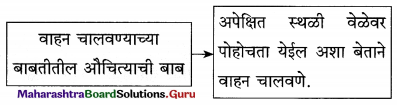
पुढील चौकटी पूर्ण करा :
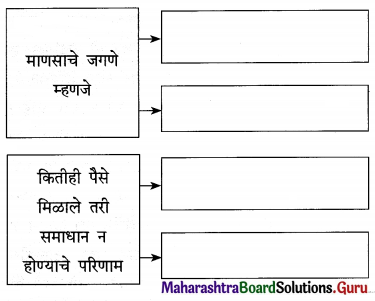
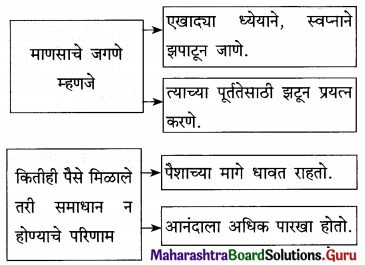
प्रश्न 1.
- एक अद्भुत सत्य [ ]
- आनंदाच्या झऱ्याच्या उगमाचे ठिकाण : [ ]
- आनंदाच्या चक्रवाढीवर फिरणारे [ ]
- एखादया ध्येयाने, स्वप्नाने झपाटणे [ ]
उत्तर :
- एक अद्भुत सत्य – आपले अस्तित्व
- आनंदाच्या झऱ्याच्या उगमाचे ठिकाण – आपले मन
- आनंदाच्या चक्रवाढीवर फिरणारे – आयुष्याचे चक्र
- एखादया ध्येयाने, स्वप्नाने झपाटणे – माणसाचे जगणे
![]()
प्रश्न 2.
- मनाची कवाडं कायमची बंद करणारा [ ]
- निरागस, आनंदी वृत्तीची [ ]
- आनंदाची इस्टेट [ ]
- आयुष्यभर न संपणारा [ ]
- शहाणंसुरतं करणारा [ ]
- कलेच्या मस्तीत जगणारे [ ]
उत्तर :
- मनाची कवाडं कायमची बंद करणारा : – दुःखी माणूस
- निरागस, आनंदी वृत्तीची : – लहान मुले
- आनंदाची इस्टेट – शास्त्रीय संगीत
- आयुष्यभर न संपणारा – शिकण्यातला आनंद
- शहाणंसुरतं करणारा – वाचनाचा छंद
- कलेच्या मस्तीत जगणारे – कलावंत
योग्य की अयोग्य ते लिहा :
प्रश्न 1.
- मनावरचे ताण नाहीसे होणे हे आनंदाचे लक्षण [ ]
- आपल्याला दृष्टी लाभली आहे, हे आपण विसरतो [ ]
- आत्म्याच्या भाषेत गाता आले नाही तरी ऐकता येऊ शकते. [ ]
- वाचन माणसाला शहाणे करते. [ ]
उत्तर :
- योग्य
- अयोग्य
- योग्य
- योग्य
पुढील वाक्याचा तुम्हांला समजलेला अर्थ लिहा :
प्रश्न 1.
आपल्या अस्तित्वाच्या आनंदाचं भान हवं.
उत्तर :
आपला श्वास, आपला दिवस-रात्र, सूर्योदय-सूर्यास्त वगैरेंकडे आपण लक्षपूर्वक कधी बघतच नाही. म्हणजे आपले अनुभव आपण लक्षपूर्वक घेत नाही. आपण ते सर्व गृहीतच धरतो. आपल्याला दृष्टी आहे, याचेही आपल्याला भान नसते. त्यामुळे आपल्याभोवती पसरलेल्या सुंदर सृष्टीचे आपल्याला कौतुक वाटत नाही. ही सृष्टी जिच्यामुळे आपल्याला दिसते, त्या आपल्या दृष्टीचेही आपल्याला कौतुक वाटत नाही. साहजिक आपले अस्तित्व आणि त्या अस्तित्वामुळे लाभलेला आनंद हे दोन्ही दुर्लक्षित राहतात.
![]()
चूक की बरोबर लिहा :
प्रश्न 1.
1. खरा आनंद दुसऱ्याच्या दुःखावर पोसला जात नाही. [ ]
2. खऱ्या आनंदात असलेल्या व्यक्तीला जग सुंदर दिसतं. [ ]
उत्तर :
1. बरोबर
2. बरोबर
हे केव्हा घडेल ते लिहा
प्रश्न 1.
दु:खासाठी आपण भरपूर कारणे शोधतो, जेव्हा …………..
उत्तर :
दुःखासाठी आपण भरपूर कारणे शोधतो, जेव्हा आपल्याला आनंद दयायला वेळच नसतो.
प्रश्न 2.
- माणसे स्वत:चा छंद कधीही विसरत नाहीत, जेव्हा …………
- तुम्ही स्वत:च्या अंत:करणात हलकेच डोकावू शकता, जेव्हा ……….
- तुम्ही वर्तमानात जगू शकता, जेव्हा ………….
उत्तर :
- माणसे स्वत:चा छंद कधीही विसरत नाहीत, जेव्हा त्याचा उद्देश केवळ आनंद मिळवणे हाच असतो.
- तुम्ही स्वत:च्या अंत:करणात हलकेच डोकावू शकता, जेव्हा तुम्ही एकटे असता.
- तुम्ही वर्तमानात जगू शकता, जेव्हा भूतकाळाची स्मृती व भविष्यकाळाची भीती या दोन्हींपासून मन मुक्त होते.
वाक्ये पूर्ण करा :
प्रश्न 1.
1. चिंता, टेन्शन यांच्या दाटीवाटीत आनंद कधीच घुसत नाही; कारण ……………..
2. लहान मुले आनंद घेण्यात तरबेज असतात; कारण ………….
उत्तर :
1. चिंता, टेन्शन यांच्या दाटीवाटीत आनंद कधीच घुसत नाही; कारण त्याला मोकळी जागा हवी असते.
2. लहान मुले आनंद घेण्यात तरबेज असतात; कारण ती निरागस व आनंदी वृत्तीची असतात.
![]()
विधाने पूर्ण करा :
प्रश्न 1.
- आपल्याला काय हवे, हे शोधणे हेच ……..
- कष्टाचे गोड हे अधिक गोड लागते, जर त्यात …………
- मुळात आनंदच शून्य असेल, तर शून्याला ………..
- आनंद जर ‘मानता’ येत असेल, तर तो …………….
उत्तर :
- आपल्याला काय हवे, हे शोधणे हेच आपण आनंदी का नाही, या प्रश्नाचे उत्तर शोधणे होय.
- कष्टाचे गोड हे अधिक गोड लागते, जर त्यात स्वकर्तृत्वाची गोडी मिसळली असेल.
- मुळात आनंदच शून्य असेल, तर शून्याला कितीही मोठ्या यशाने किंवा पैशाने गुणले तरी गुणाकार शून्यच.
- आनंद जर ‘मानता’ येत असेल, तर तो ‘मिळवण्याचा’ प्रयत्न कशाला करायचा?
अलंकार :
पुढील ओळींमधील अलंकार ओळखा :
प्रश्न 1.
1. हे हृदय नसे, परि स्थंडिल धगधगलेले → [ ]
2. काव्य अगोदर झाले नंतर जग झाले सुंदर, रामायण आधी मग झाला राम जानकीवर → [ ]
उत्तर :
1. अपन्हुती अलंकार
2. अतिशयोक्ती अलंकार
आयुष्य… आनंदाचा उत्सव Summary in Marathi
पाठ परिचय :
प्रस्तुत पाठ म्हणजे ‘मजेत जगावं कसं?’ या गाजलेल्या पुस्तकातील एक लेख आहे. जीवन आनंदात कसे जगावे, हे सांगण्याचा या लेखात लेखकांनी प्रयत्न केला आहे.
आनंद हा यांत्रिकपणे, खूप प्रयत्न करून किंवा पैसे देऊन मिळत नाही. स्वतःचे मन, अंत:करण आनंदी ठेवले पाहिजे. तरच आनंद मिळतो. स्वत:च्या मनातील सर्व किल्मिषे, सर्व नकारात्मक भाव काढून टाकले, तर मन शुद्ध होते. शुद्ध मन हाच आनंदाचा स्रोत असतो.
कला, साहित्य व निसर्गसहवास यांच्या माध्यमातून आपण स्वत:चे मन शुद्ध करू शकतो. ही क्षेत्रे आनंदाला पूरक अशी मनोवृत्ती निर्माण करतात.
शब्दार्थ :
- शाश्वत – चिरकालिक, चिरंतन, अविनाशी.
- कळसा – नळ लावलेली मातीची घागर.
- निखळ – पवित्र, शुद्ध, निर्भेळ.
- ईर्षा – चुरस, चढाओढ, हेवा.
- असूया – द्वेष, मत्सर.
- वैषम्य – खेद, दुःख, विषमता.
- कवाडे – घराची किंवा खिडक्यांची दारे.
- जडणे – सांधणे, कोंदणात बसवणे.
![]()
वाक्प्रचार व त्यांचे अर्थ :
- आटापिटा करणे – खटाटोप करणे, खूप कष्टाने प्रयत्न करणे.
- मनाची कवाडे बंद करणे – मन मोकळे न ठेवणे, पूर्वग्रहदूषित वृत्ती बाळगणे.
- (एखाद्या गोष्टीत) रंगून जाणे – विलीन होण, पूर्णपणे मिसळून जाणे.


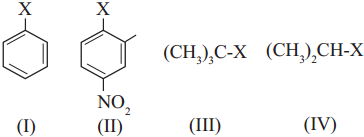



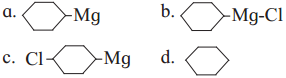

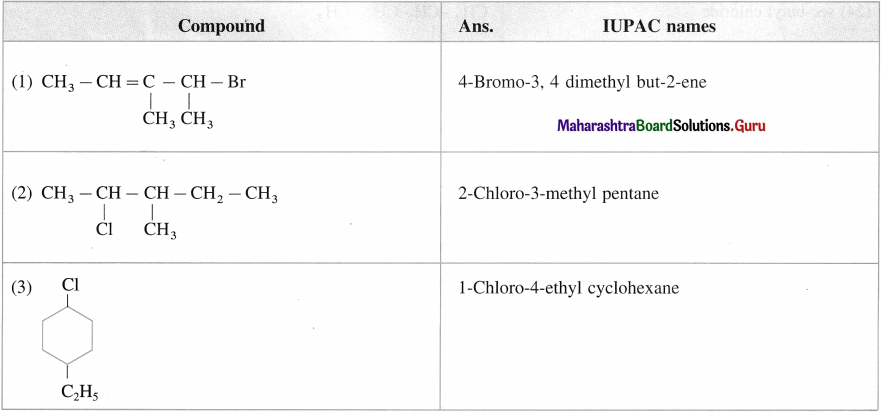

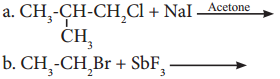




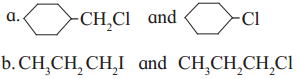
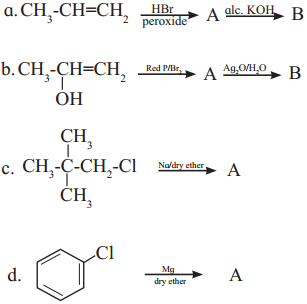
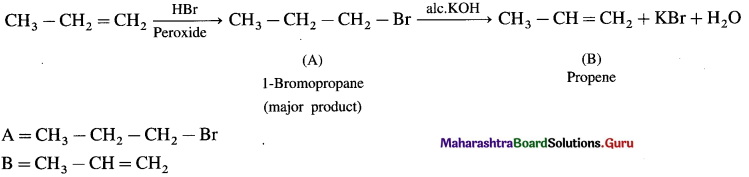
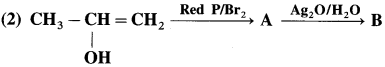
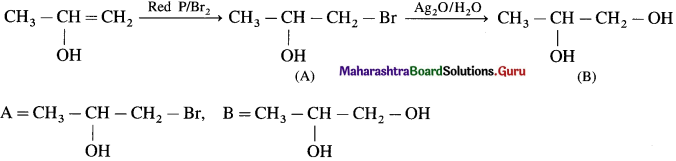
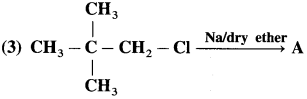
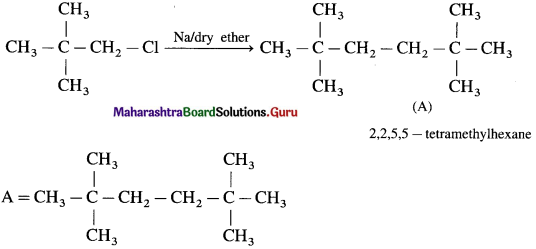
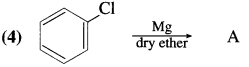




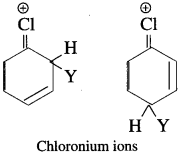
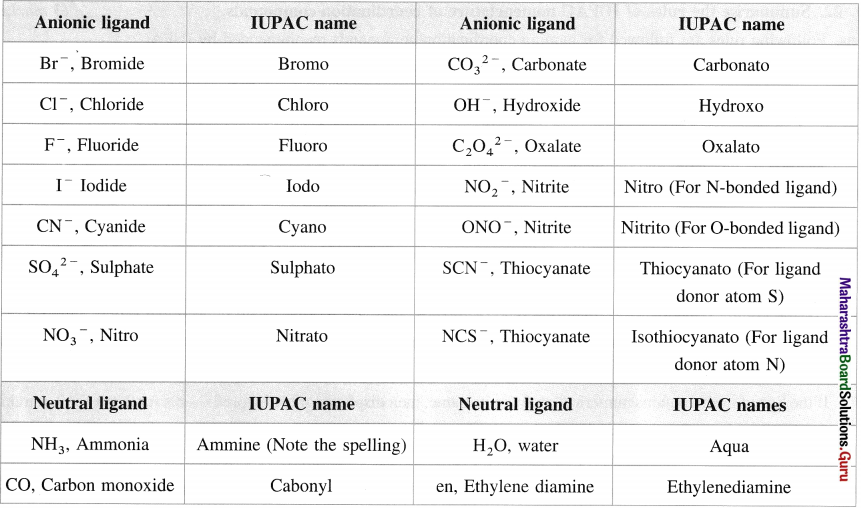
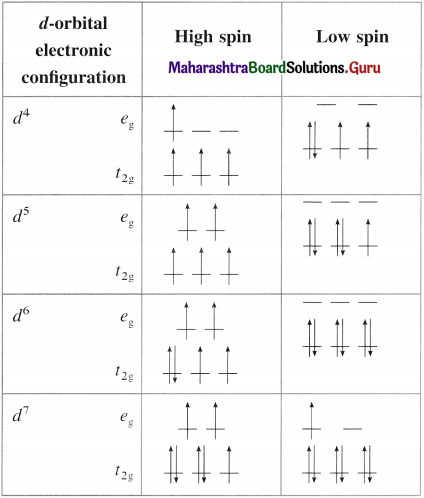
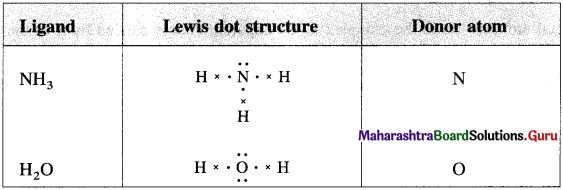
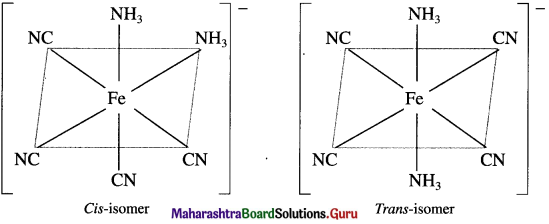
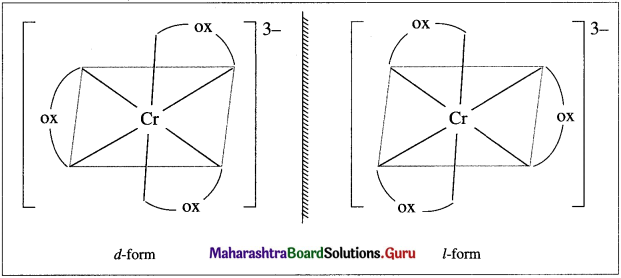
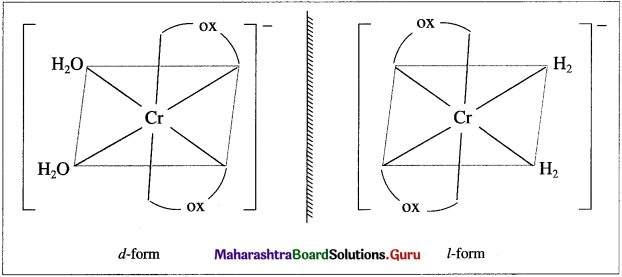
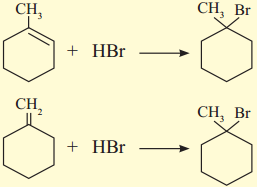
 has mp. higher than those of o-and rn-isomers.
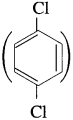
has mp. higher than those of o-and rn-isomers.
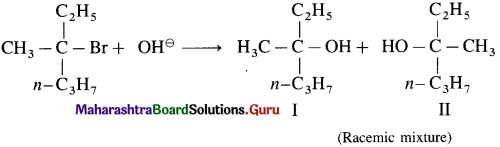
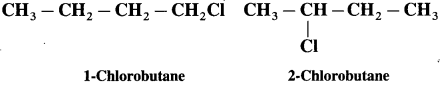
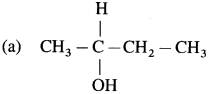
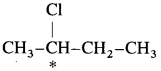 carbon atom (the starred carbon atom) which is attached to four different groups, i.e., ethyl (-CH2 – CH3), methyl (CH3), chloro (Cl) and hydrogen (H) groups.
carbon atom (the starred carbon atom) which is attached to four different groups, i.e., ethyl (-CH2 – CH3), methyl (CH3), chloro (Cl) and hydrogen (H) groups.
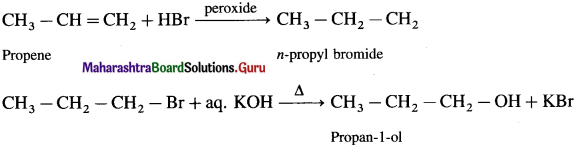


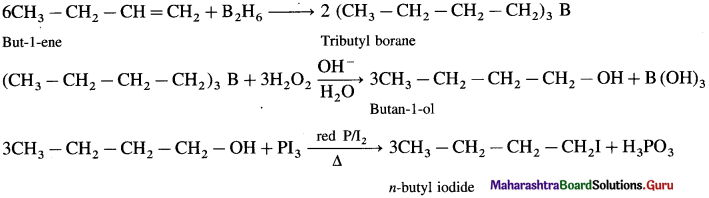
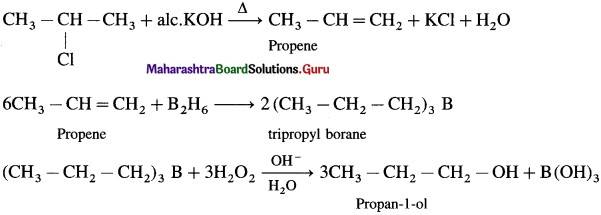


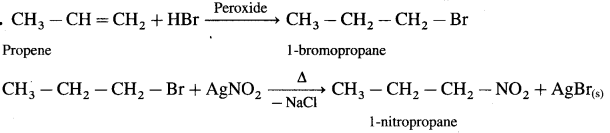


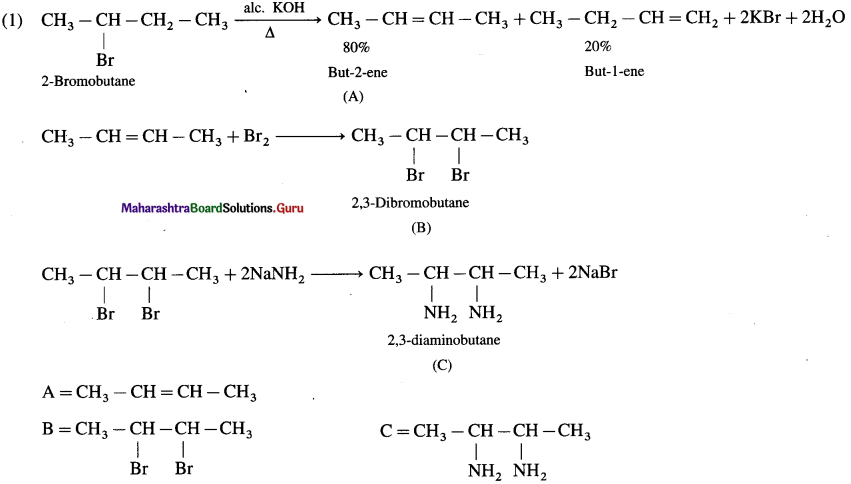
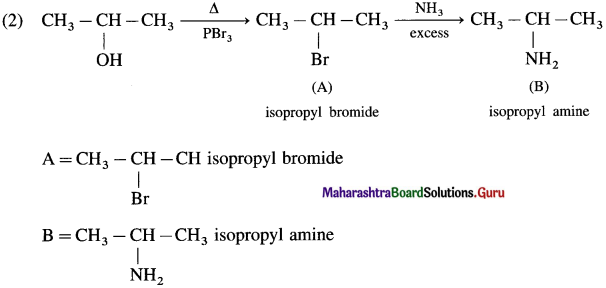
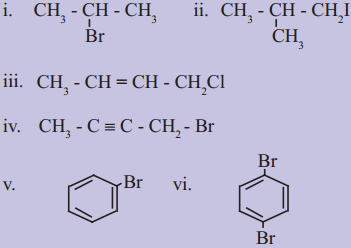
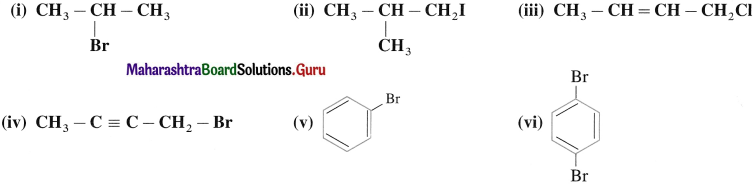
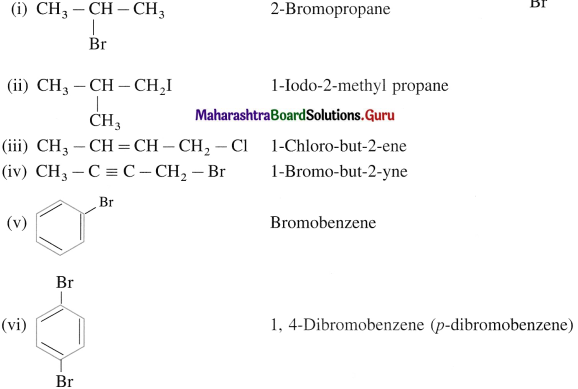



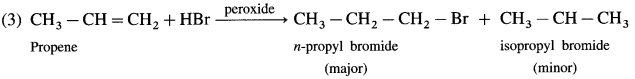
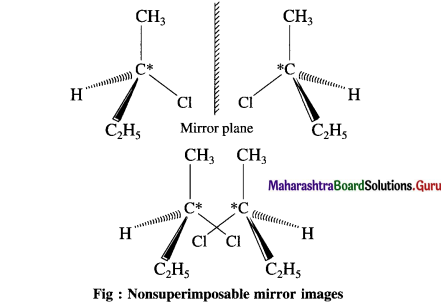
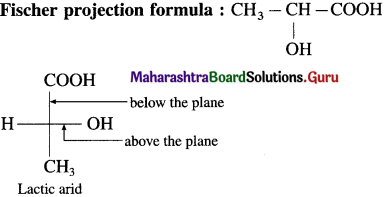
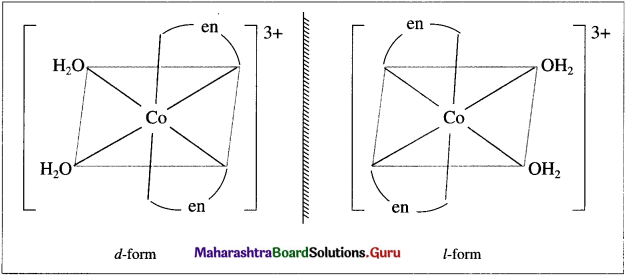
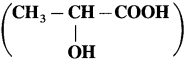 using Fischer projection formulae.
using Fischer projection formulae.