Std 11 Hindi Chapter 1 Prerna Question Answer Maharashtra Board
Balbharti Maharashtra State Board Hindi Yuvakbharati 11th Digest Chapter 1 प्रेरणा Notes, Textbook Exercise Important Questions and Answers.
Hindi Yuvakbharati 11th Digest Chapter 1 प्रेरणा Questions And Answers
11th Hindi Digest Chapter 1 प्रेरणा Textbook Questions and Answers
कृति-स्वाध्याय एवं उत्तर
आकलन
1. सूचनाओं के अनुसार कृतियाँ कीजिए :
प्रश्न अ.
कारण लिखिए –
(a) माँ, मेरी आवाज सुनकर रोती है –
उत्तर :
माँ, मेरी आवाज सुनकर रोती है क्योंकि उसकी ममता आँसुओं के रूप में फूट पड़ती है।
![]()
(b) बच्चों को माता-पिता का प्यार टुकड़ों में मिलता है –
उत्तर :
बच्चों को माता-पिता का प्यार टुकड़ों में मिलता है क्योंकि माता-पिता पर काम पर जाने की मजबूरी है।
(c) कवि की उम्र बढ़ती ही नहीं है –
उत्तर :
कवि की उम्र बढ़ती ही नहीं है क्योंकि उसने अपनी आँखों में स्वयं को एक बालक के रूप में पाया।
प्रश्न आ.
लिखिए –
(a)

उत्तर :

काव्य सौंदर्य
2.
प्रश्न अ.
ममत्व का भाव प्रकट करने वाली कोई भी एक त्रिवेणी ढूँढ़कर उसका अर्थ लिखिए।
उत्तर :
‘माँ मेरी बे-वजह ही रोती है
फोन पर जब भी बात होती है
फोन रखने पर मैं भी रोता हूँ।’
प्रस्तुत त्रिवेणी में ममत्व का भाव प्रकट हुआ है। जब कभी कवि अपनी माँ को फोन करता है तब पुत्र की आवाज सुनकर माँ की ममता रो पड़ती है। कवि भले ही इसे बे-वजह रोना कहता है, परंतु सच्चाई तो यही है कि कवि भी फोन रखने के बाद रोता है क्योंकि माता और पुत्र दोनों एक-दूसरे के स्नेह के लिए तरसते हैं और उनका एक-दूसरे के प्रति स्नेह आँसुओं के रूप में आँखों से बहने लगता है।
![]()
प्रश्न आ.
निम्न पंक्तियों में से प्रतीकात्मक पंक्ति छाँटकर उसे स्पष्ट कीजिए –
(a) चलते-चलते जो कभी गिर जाओ
(b) रात की कोख ही से सुबह जनम लेती है
(c) अपनी आँखों में जब भी देखा है
उत्तर :
‘रात की कोख से सुबह जनम लेती है’ यह पंक्ति प्रतीकात्मक (symbolic) पंक्ति है। यहाँ रात दुख एवं अँधेरे का प्रतीक है और सुबह सुख एवं उजाले का प्रतीक है। सुख और दुख का आना-जाना दिन और रात के समान है। दोनों स्थायी रूप से जीवन में कभी नहीं रहते। जैसे रात के बाद दिन का आना निश्चित है वैसे ही दुख के बाद सुख का आना भी निश्चित है। अत: दुखसुख दोनों का सहर्ष (readily) स्वागत करते हुए जिंदगी में आगे बढ़ना चाहिए।
अभिव्यक्ति
3.
प्रश्न अ.
पालनाघर की आवश्यकता पर अपने विचार लिखिए।
उत्तर :
आज कामकाजी महिलाओं को घर से बाहर जाना पड़ता है और यह समय की माँग भी है कि पति-पत्नी दोनों अपने परिवार नैया की पतवार सँभालें। ऐसी स्थिति में इनके छोटे बच्चों को परेशानी होती है। कभी-कभी इसका परिणाम इनके कार्य पर भी पड़ता है। ऐसे में कामकाजी महिलाओं के बच्चों के लिए पालनाघर की सुविधा आवश्यक है। पालनाघर में बच्चों को घर जैसी देखरेख और सुरक्षा मिलती है।
अगर पालनाघर में बच्चे को घर जैसा माहौल मिलता हो तो महिलाएँ निश्चित होकर आत्मनिर्भर बनने के लिए कदम उठा पाती हैं। भारत की आधी आबादी महिलाओं की है। अत: महिलाएँ अगर श्रम में योगदान दें तो भारत का विकास तेजी से होगा। पालनाघर की सुविधा मुहैया कराने से महिलाओं की भागीदारी बढ़ेगी और देश के विकास पर इसका असर अवश्य दिखाई देगा। उन्हें बच्चों की देखभाल करने के लिए काम छोड़ने की नौबत नहीं आएगी।
घरेलु आमदनी में भी इजाफा (increase) होगा। परिणामत: बच्चों की सेहत और शिक्षा में भी सुधार होगा। संक्षेप में महिलाओं का काम करना महत्त्वपूर्ण है ताकि उन्हें अलग पहचान मिले और इसके लिए पालनाघर की सुविधा अगर उनके कार्य-स्थल के आस-पास हो तो सोने पे सुहागा हो सकता है।
प्रश्न आ.
नौकरीपेशा अभिभावकों के बच्चों के पालन की समस्या पर प्रकाश डालिए।
उत्तर :
आजकल माता-पिता दोनों का नौकरी करना बहुत ही आम बात है। ऐसे में उनके बच्चों के पालन की समस्या उभर आती है। बच्चों की तरफ पूरा-पूरा ध्यान दे पाना उनके लिए मुश्किल होता है। नौकरी और बच्चों का लालन-पालन दोनों में संतुलन बनाना कठिन हो जाता है।
बच्चों की परवरिश उनके लिए एक समस्या बन जाती है। वास्तव में कामकाजी माता-पिता के पास अक्सर समय की कमी होती है। समय का उचित नियोजन करके इस समस्या से निजात (escape) पाया जा सकता है। अपने छोटे-छोटे कार्यों में बच्चों को सम्मिलित कर लेने से बच्चों को भी अहमियत मिल जाती है और बच्चे जिम्मेदार भी बन जाते हैं। घर का समय बच्चों को देकर उन्हें खुश रखा जा सकता है।
![]()
उन्हें अच्छी आदतें सिखाने का समय मिल जाता है। माता-पिता के जीवन में बच्चे महत्त्वपूर्ण हैं इस बात का एहसास दिलाया जा सकता है। अक्सर कामकाजी माता-पिता के बच्चों के मन में यह भावना पनपती (flourish) रहती है कि माता-पिता के लिए काम ही महत्त्वपूर्ण है और वे बच्चों से प्यार नहीं करते। कई बार दफ्तर की परेशानियाँ घर तक ले आते हैं और अपना क्रोध वे बच्चों पर निकाल देते हैं।
ऐसे में डर के कारण बच्चे अभिभावकों से दूर हो जाते हैं। इस स्थिति से बचने की कोशिश अभिभावक अवश्य करें। बच्चों की बातें चाव से सुनना, दफ्तर की बातें उन्हें बताना, साथ में भोजन करना, छुट्टी के दिन घूमने ले जाना जैसी छोटी-छोटी बातें भी कामकाजी अभिभावक और बच्चों का रिश्ता मजबूत करती हैं। इस प्रकार समस्या हैं तो उसके उपाय भी है जिन पर अवश्य अमल करना चाहिए।
रसास्वादन
4. आधुनिक जीवन शैली के कारण निर्मित समस्याओं से जूझने की प्रेरणा इन त्रिवेणियों से मिलती है, स्पष्ट कीजिए।
उत्तर :
(i) शीर्षक : प्रेरणा
(ii) रचनाकार : त्रिपुरारि
(iii) केंद्रीय कल्पना : अर्थ प्रधान एवं अतिव्यस्त आधुनिक जीवन शैली अपनाने के कारण आज मनुष्य न चाहते हुए भी अकेला पड़ गया है। तनाव में जी रहा है। मानसिक असंतुलन, चिंता, उदासी, सूनापन आदि से चारों ओर से घिरा है। भाग-दौड़ भरी जिंदगी में आगे निकलने की होड़ लगी हैं और उसके रिश्ते पीछे छूट रहे हैं। जिंदगी की आपाधापी में जुटे माता-पिता के बच्चों को स्नेह भी टुकड़ों में मिलता है।
जीवन की इन समस्याओं को उजागर करते हुए त्रिपुरारि जी ने अपनी त्रिवेणियों में माँ के ममत्व और पिता की गरिमा को भी व्यक्त किया है। जीवन में आगे निकलने की होड़ में चोट खाकर गिरने पर भी सँभलकर फिर से चलने की, आगे बढ़ने की सलाह दी है।
(iv) रस-अलंकार : तीन पंक्तियों के मुक्त छंद में इस कविता की त्रिवेणियाँ लिखी हुई हैं।
(v) प्रतीक विधान : जीवन के प्रति सकारात्मकता का विधान है – पेड़ में बीज और बीज में पेड़ छुपा है। यहाँ बीज हमारी भावनाओं का प्रतीक है और उसे आँसुओं के जल से सींचकर खुशियों का वृक्ष फलता फूलता है।
(vi) कल्पना : खुद के भीतर छिपे बालक को जीवित रखने की कल्पना हमें तनाव, कुंठा से मुक्ति देगी।
(vii) पंसद की पंक्तियाँ तथा प्रभाव :
‘चाहे कितनी ही मुश्किलें आएँ
छोड़ना मत उम्मीद का दामन
नाउम्मीदी तो मौत है प्यारे।’
यह त्रिवेणी मुझे पसंद है क्योंकि सचमुच कठिनाइयों से घबराकर निराश व्यक्ति जीते जी मर जाता है। हर अंधेरी काली रात के बाद सुबह उजाला लेकर अवश्य आता है। ठीक इसी तरह दुख के बाद सुख का आना निश्चित है इस उम्मीद के साथ जीना चाहिए। यही जीवन के प्रति सकारात्मकता है।
(viii) कविता पसंद आने के कारण : सामयिक स्थितियाँ, रिश्ते और जीवन के प्रति सकारात्मकता कविता का मुख्य विषय है जो प्रेरणादायी है। त्रिपुरारि जी की ये त्रिवेणियाँ हमें जीने की कला सिखाती है, इसीलिए मुझे कविता पसंद है।
साहित्य संबंधी सामान्य ज्ञान
5. जानकारी दीजिए:
![]()
प्रश्न अ.
त्रिवेणी’ काव्य प्रकार की विशेषताएँ –
(a) ……………………………………………..
(b) ……………………………………………..
उत्तर :
(a) तीन पंक्तियों का मुक्त छंद है।
(b) मात्र तीन पंक्तियों में कल्पना और यथार्थ (reality) की अभिव्यक्ति (expression) होती है।
प्रश्न आ.
त्रिपुरारि जी की अन्य रचनाएँ –
(a) ……………………………………………..
(b) ……………………………………………..
उत्तर :
(a) नींद की नदी (कविता संग्रह)
(b) नॉर्थ कैंपस (कहानी संग्रह)
रस
काव्यशास्त्र में आचार्यों ने रस को काव्य की आत्मा माना है। विभाव, अनुभाव, व्यभिचारी (संचारी) भाव और स्थायी भाव रस के अंग हैं और इन अंगों अर्थात तत्त्वों के संयोग से रस की निष्पत्ति होती है।
साहित्यशास्त्र में नौ प्रकार के रस माने गए हैं। कालांतर में अन्य दो रसों को सम्मिलित किया गया है।
| रस | स्थायी भाव |
| (१) शृंगार | प्रेम |
| (७) भयानक | भय |
| (२) शांत | शांति |
| (८) बीभत्स | घृणा |
| (३) करुण | शोक |
| (९) अद्भुत | आश्चर्य |
| (४) हास्य | हास |
| (१०) वात्सल्य | ममत्व |
| (५) वीर | उत्साह |
| (११) भक्ति | भक्ति |
| (६) रौद्र | क्रोध |
![]()
करुण रस :
किसी प्रियजन या इष्ट के कष्ट, शोक, दुख, मृत्युजनित प्रसंग के कारण अथवा किसी प्रकार की अनिष्ट आशंका के फलस्वरूप हृदय में पीड़ा या क्षोभ का भाव उत्पन्न होता है, वहाँ करुण रस की अभिव्यंजना होती है।
उदा. –
(१) वह आता –
दो टूक कलेजे के करता पछताता पथ पर आता।
पेट – पीठ दोनों मिलकर हैं एक,
– मैथिलीशरण गुप्त
(२) अबला जीवन हाय ! तुम्हारी यही कहानी,
आँचल में है दूध और आँखों में पानी।
चल रहा लकुटिया टेक
मुट्ठी भर दाने को भूख मिटाने को,
मुँह फटी झोली फैलाता।
– सूर्यकांत त्रिपाठी ‘निराला’
Yuvakbharati Hindi 11th Textbook Solutions Chapter 1 प्रेरणा Additional Important Questions and Answers
कृतिपत्रिका
(अ) निम्नलिखित पठित पद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| पद्यांश : माँ मेरी बे-वजह ………………………………………… टुकड़ों में मिले हैं माँ-बाप (पाठ्यपुस्तक पृष्ठ क्र. 1) |
प्रश्न 1.
कारण लिखिए :
(i) माँ, मेरी आवाज सुनकर रोती है –
उत्तर :
माँ, मेरी आवाज सुनकर रोती है क्योंकि उसकी ममता आँसुओं के रूप में फूट पड़ती है।
(ii) बच्चों को माता-पिता का प्यार टुकड़ों में मिलता है –
उत्तर :
बच्चों को माता-पिता का प्यार टुकड़ों में मिलता है क्योंकि माता-पिता पर काम पर जाने की मजबूरी है।
प्रश्न 2.
पर्यायवाची शब्द लिखिए :
उत्तर :
(i) माँ = …………………………. , ………………………….
उत्तर :
जननी, माता, धात्री
(ii) रात = …………………………. , ………………………….
उत्तर :
रजनी, निशा, यामिनी
![]()
प्रश्न 3.
पद्यांश की द्वितीय त्रिवेणी का भावार्थ लिखिए।
उत्तर :
प्रस्तुत पद्यांश कवि त्रिपुरारि जी लिखित त्रिवेणी संग्रह ‘साँस के सिक्के’ से लिया गया है। इस त्रिवेणी में पिता की गरिमा व्यक्त करते हुए कवि कह रहे हैं कि पिता को कठोर हृदय का समझने की भूल हम सब करते हैं। परंतु सच्चाई यही है कि वह ऊपर से जितने सख्त नजर आते हैं भीतर से उतने ही कोमल होते हैं।
जब संतान को चोट लगती है तो पिता का कोमल हृदय भी तड़प उठता है। हर पिता में इस तरह कोई माँ भी छुपी होती है अर्थात पिता भी अपनी संतान से उतना ही प्यार करता है, जितना कि एक माँ। दोनों के स्नेह में कोई तुलना नहीं होनी चाहिए।
(आ) निम्नलिखित पद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| पद्यांश : चाहे कितना भी हो घनघोर अंधेरा ……………………………………………… जहाँ पर भी कदम रखोगे। (पाठ्यपुस्तक पृष्ठ क्र. 2) |
प्रश्न 1.
परिणाम लिखिए :
(i) अँधेरा होने पर भी आस रखने का परिणाम
उत्तर :
अँधेरा होने पर भी आस रखने से किसी दिन उजाला अवश्य होता है।
(i) एक ही दीप से आगाज-ए-सफर कर लेने का परिणाम –
उत्तर :
एक ही दीप से आगाज-ए-सफर कर लेने से जहाँ भी कदम रखते हैं वहाँ रोशनी हो जाती है।
प्रश्न 2.
पद्यांश से विलोम शब्द की जोड़ियाँ ढूँढ़कर लिखिए :
उत्तर :
(i) …………………………… x ……………………………
अंधेरा x उजाला x …….
(ii) …………………………… x ……………………………
पास x दूर
प्रश्न 3.
पद्यांश की किसी एक त्रिवेणी का काव्य सौंदर्य लिखिए :
उत्तर :
प्रस्तुत पद्यांश ‘प्रेरणा’ कविता से लिया गया है। कवि त्रिपुरारि जी लिखित साँस के सिक्के ‘त्रिवेणी संग्रह’ में ये त्रिवेणियाँ संकलित (consalidated) हैं। दिए गए पद्यांश की द्वितीय त्रिवेणी में वीर रस की निर्मिति (build up) हुई है। प्राप्त परिस्थितियों में भी उत्साह के साथ आगे बढ़ने की कोशिश यहाँ दिखाई देती है। अपनी आयु के कुछ पल उधार लेकर ही सही हसरत के बीज बोने का साहस करके सपनों को साकार करने की बात वीरतापूर्ण है। निराशा के बादलों के बीच आशा का संचार इस त्रिवेणी की विशेषता है।
![]()
(इ) निम्नलिखित पठित पद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| पद्यांश : अपनी आँखों में जब भी …………………………………………….. नाउम्मीदी तो मौत है प्यारे। (पाठ्यपुस्तक पृष्ठ क्र. 2) |
प्रश्न 1.
उचित जोड़ियाँ मिलाइए :
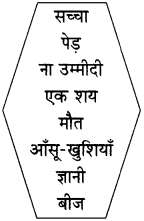
| ‘अ’ | ‘आ’ |
| (1) …………………………… | …………………………… |
| (2) …………………………… | …………………………… |
| (3) …………………………… | …………………………… |
| (4) …………………………… | …………………………… |
उत्तर :
| ‘अ’ | ‘आ’ |
| (1) सच्चा | ज्ञानी |
| (2) पेड़ | बीज |
| (3) ना उम्मीदी | मौत |
| (4) एक शय | आँसू-खुशियाँ |
प्रश्न 2.
पद्यांश की प्रथम त्रिवेणी का भावार्थ लिखिए।
उत्तर :
प्रस्तुत पद्यांश त्रिवेणी विधा में लिखी कविता ‘प्रेरणा’ से लिया गया है। कवि त्रिपुरारि जी ने पद्यांश की प्रथम त्रिवेणी में हमारे अंदर छिपे बालक को जीवित रखने की सलाह दी है। उन्होंने जब भी स्वयं की आँखों में देखा स्वयं को एक बच्चे की तरह ही पाया और उन्हें लगा कि दुनिया उन्हें बड़ा बनाकर दुनियादारी निभाने पर मजबूर करती है।
इसकी वजह से जीवन की अबोधता (innocence), मासूमियत नष्ट हो जाती है और मन अशांत हो जाता है। इससे छुटकारा पाना है तो कभी अपने भीतर छिपे बालक को मरने नहीं देना चाहिए।
![]()
स्वाध्याय
आकलन : 1. सूचनाओं के अनुसार कृतियाँ कीजिए :
(अ) कारण लिखिए –
प्रश्न 1.
घनघोर अँधेरे में भी उजाले की आस रखनी चाहिए –
उत्तर :
घनघोर अँधेरे में भी उजाले की आस रखनी चाहिए क्योंकि रात की कोख से ही सुबह का जन्म होता है।
प्रश्न 2.
उम्मीद का दामन नहीं छोड़ना चाहिए –
उत्तर :
उम्मीद का दामन नहीं छोड़ना चाहिए क्योंकि नाउम्मीदी मौत के समान है।
(आ) लिखिए –
उत्तर:
काव्य सौंदर्य :
व्याकरण
रस :
काव्यशास्त्र (poetics) में आचार्यों ने रस को काव्य की आत्मा माना है। विभाव, अनुभाव, व्यभिचारी (संचारी) भाव और स्थायी भाव रस के अंग हैं और इन अंगों अर्थात तत्त्वों के संयोग से रस की निष्पत्ति होती है। साहित्यशास्त्र में नौ प्रकार के रस माने गए हैं। कालांतर (afterward) में अन्य दो रसों को सम्मिलित किया गया है।
| रस | स्थायी भाव |
| (1) शृंगार | प्रेम |
| (2) शांत | शांति |
| (3) करुण | शोक |
| (4) हास्य | हास |
| (5) वीर | उत्साह |
| (6) रौद्र | क्रोध |
| (7) भयानक | भय |
| (8) बीभत्स | घृणा |
| (9) अद्भुत | आश्चर्य |
| (10) वात्सल्य | ममत्व |
| (11) भक्ति | भक्ति |
![]()
1. शृंगार रस :
इसे रसराज कहा गया है। यह संयोग और वियोग इन दो भागों में विभाजित है। जब किसी काव्य में नायक नायिका के प्रेम, मिलने-बिछड़ने आदि का वर्णन होता है तो वह शृंगार रस कहलाता है।
उदा.
(i) रे मन आज परीक्षा मेरी।
सब अपना सौभाग्य मनावें।
दरस परस नि:श्रेयस पावें।
उद्धारक (redeemer) चाहें तो आवें।
यही रहे यह चेरी। – मैथिलीशरण गुप्त
(ii) बतरस लालच लाल की मुरली धरि लुकाय।
सौंह करै, भौहनि हँस, दैन कहै, नटि जाय।
– बिहारी
2. शांत रस :
जब कभी काव्य को पढ़कर मन में असीम शांति का एवं दुनिया से मोह खत्म होने का भाव उत्पन्न हो तो शांत रस की अनुभूति होती है। ज्ञान की प्राप्ति और संसार से वैराग्य होने पर शांत रस की उत्पत्ति होती है।
उदा.
(i) जब मैं था हरि नाहिं अब हरि है मैं नाहिं
सब अँधियारा मिट गया जब दीपक देख्या माहिं
– कबीर
(ii) देखी मैंने आज जरा!
हो जावेगी क्या ऐसी मेरी यशोधरा?
हाय! मिलेगा मिट्टी में वह वर्ण सुवर्ण खरा?
सूख जावेगा मेरा उपवन जो है आज हरा?
– मैथिलीशरण गुप्त
3. करुण रस :
किसी प्रियजन या इष्ट के कष्ट, शोक, दुख, मृत्युजनित प्रसंग के कारण अथवा किसी प्रकार की अनिष्ट (undesired) आशंका के फलस्वरूप हृदय में पीड़ा या क्षोभ का भाव उत्पन्न होता है, वहाँ करुण रस की अभिव्यंजना (expression) होती है।
उदा.
(i) वह आता –
दो टूक कलेजे के करता पछताता पथ पर आता।
पेट-पीठ दोनों मिलकर हैं एक,
चल रहा लकुटिया टेक
मुट्ठी भर दाने को, भूख मिटाने को, मुँह फटी झोली फैलाता।
– सूर्यकांत त्रिपाठी ‘निराला’
![]()
(ii) अबला जीवन हाय ! तुम्हारी यही कहानी,
आँचल में है दूध और आँखों में पानी।
– मैथिलीशरण गुप्त
4. हास्य रस :
जब किसी काव्य को पढ़कर हँसी आ जाती है तब हास्य रस की उत्पत्ति होती है। सभी रसों में यह रस सबसे अधिक सुखात्मक रस है।
उदा.
(i) हाथी जैसा देह, गेंडे जैसी चाल।
तरबूजे सी खोपड़ी, खरबूजे से गाल।।
(ii) बुरे समय को देखकर गंजे तू क्यों रोय।
किसी भी हालत में तेरा बाल न बाँका होय।।
5. वीर रस :
शत्रु को मिटाने, दीन-दुखियों का उद्धार करने, धर्म की स्थापना, उद्धार करने आदि में जो मन में उत्साह उमड़ता है वही वीर रस की उत्पत्ति होती है।
उदा.
(i) सच है, विपत्ति जब आती है।
कायर को ही दहलाती (horror) है,
सूरमा नहीं विचलित होते,
क्षण एक नहीं धीरज खोते,
कर शपथ विघ्नों को गले लगाते हैं,
काँटों में राह बनाते हैं।
– रामधारी सिंह दिनकर
(ii) तू न थकेगा कभी,
तू न रुकेगा कभी,
तू न मुड़ेगा कभी,
कर शपथ, कर शपथ,
अग्निपथ, अग्निपथ, अग्निपथ।
– हरिवंशराय बच्चन
![]()
6. रौद्र रस :
अपमान, निंदा आदि से मन में क्रोध उत्पन्न होता है तब रौद्र रस की उत्पत्ति होती है।
उदा.
(i) अस कहि रघुपति चाप बढ़ावा,
यह मत लछिमन के मन भावा।
संधानेहु प्रभु बिसिख कराला,
उठि ऊदधि उर अंतर ज्वाला।।
– तुलसीदास
(ii) उस काल मारे क्रोध के तन काँपने उसका लगा
मानो हवा के जोर से सोता हुआ सागर जगा।
मैथिलीशरण गुप्त
7. भयानक रस :
भयोत्पादक वस्तुओं को देखने या सुनने से अथवा शत्रु आदि के अनिष्ठ आचरण से भयानक रस की
निष्पत्ति होती है। इसका स्थायी भाव भय है। अंतर्गत कंपन, पसीना छूटना, मुँह सूखना, चिंता आदि भाव भय के कारण उत्पन्न होते हैं।
उदा.
(i) अखिल यौवन के रंग उभार, हड्डियों के हिलते कंकाल
कचो के चिकने काले, व्याल, केंचुली, काँस, सिबार।
– सुमित्रानंदन पंत
(ii) एक ओर अजगर हिं लखि, एक ओर मृगराय
विकल बटोही बीच ही, पर्यो मूरछा खाय।
8. बीभत्स रस :
वीभत्स यानि घृणा इसका स्थायी भाव है। घृणित वस्तुओं घृणित चीजों या घृणित व्यक्तियों को देखकर, उनके संबंध में सुनकर मन में उत्पन्न होने वाली घृणा या ग्लानी बीभत्स रस को पुष्टि करती है।
उदा.
(i) आँखें निकाल उड़ जाते, क्षण भर उड़कर आ जाते
शव जीभ खींचकर कौए, चुभला-चभलाकर खाते
भोजन में श्वान लगे मुरदे थे भू पर लेटे
खा माँस चाट लेते थे, चटनी सैम बहते बहते बेटे
– श्यामनारायण पांडेय
![]()
(ii) विष्टा पूय रुधिर कच हाडा
बरबई कबहु उपल बहु छाडा।
– तुलसीदास
9. अद्भुत रस :
इसका स्थायी भाव आश्चर्य है। जब व्यक्ति के मन में विचित्र अथवा आश्चर्यजनक वस्तुओं को देखकर विस्मय उत्पन्न होता है तो अद्भुत रस की निष्पत्ति होती है।
उदा.
(i) देख यशोदा शिशु के मुख में सकल विश्व की माया
क्षणभर को वह बनी अचेतन (unconscious), हिल न सकी कोमल काया।
– सूरदास
(ii) पड़ी अचानक नदी अपार, घोड़ा कैसे उतरे पार।
राणा ने सोचा इस पार तब तक चेतक था उस पार।।
– श्यामनारायण पांडेय
10. वात्सल्य रस :
माता का पुत्र के प्रति प्रेम, बड़ों का बच्चों के प्रति प्रेम, गुरु का शिष्य के प्रति प्रेम की अभिव्यक्ति से वात्सल्य रस की निष्पत्ति होती है। ममत्व, अनुराग, स्नेह इस रस का स्थायी भाव है।
उदा.
(i) वह मेरी गोदी की शोभा, सुख सुहाग की है लाली,
शाही शान भिखारिन की है मनोकामना मतवाली।
– सुभद्राकुमारी चौहान
(ii) सुत मुख देखि जसोदा फूली।
हरषति देख दूध की द॑तिया,
प्रेम मगन तन की सुधि भूली।
– सूरदास
![]()
11. भक्ति रस :
इस रस में ईश्वर के प्रति अनुराग का, प्रेम का वर्णन होता है। श्रद्धा, स्मृति, मति, धृति, उत्साह आदि भाव इसमें समाविष्ट होते हैं। भक्ति भाव के मूल में दास्य, साख्य, वात्सल्य, माधुर्य भक्ति भाव समाविष्ट हैं।
उदा.
(i) अँसुवन जल सिंची-सिंची प्रेम-बेलि बोई
मीरा की लगन लागी, होनी हो सो होई
– मीरा
(ii) कहत कबीर सुनहु रे लाई
हरि बिन राखनहार न कोई।
– कबीर
प्रेरणा Summary in Hindi
प्रेरणा कवि परिचय :

एरौत (बिहार) (5 दिसंबर, 1988) में जन्मे त्रिपुरारि कुमार शर्मा जी एक कवि, लेखक, संपादक (editor) और अनुवादक (translater) हैं। शिक्षा, रचनात्मक लेखन और पत्रकारिता के क्षेत्र में सफलतापूर्वक कार्य करते हुए ‘त्रिवेणी’ काव्य (poetry) रचना में अपनी विशेष पहचान बना चुके हैं। नींद की नदी’, ‘नॉर्थ कैंपस, साँस के सिक्के आदि आपकी प्रमुख कृतियाँ हैं। विभिन्न पत्र-पत्रिकाओं में आपकी कविताएँ, कहानियाँ, लेख आदि प्रकाशित हुए हैं।
प्रेरणा कविता का परिचय :
प्रस्तुत रचना ‘त्रिवेणी’ विधा में लिखी कविता है। ‘त्रिवेणी’ एक तीन पंक्तियों वाली कविता है जिसे प्रसिद्ध गीतकार और कवि गुलजार साहब ने विकसित (develop) किया है। शब्दों की किफायत और एहसासों की अमीरी इस विधा की विशेषता है। एक चलचित्र की तरह इसकी पहली दो पंक्तियाँ कहानी को गढ़ती है और तीसरी पंक्ति कहानी की परिपूर्णता को अभिव्यक्त करते हुए क्लाइमेक्स व्यक्त कराती है। प्रस्तुत त्रिवेणियों में त्रिपुरारि जी ने कहीं माँ की ममता, पिता का गौरव तो कहीं जीवन की कठोर सच्चाई को व्यक्त करते हुए मुश्किलों का डटकर सामना करने की सलाह दी है और उम्मीद का दामन पकड़कर आगे बढ़ने का मशवरा दिया है।
प्रेरणा कविता का भावार्थ (purport) :
जीवन की आपाधापी में माता-पुत्र बिछड़ जाते हैं। दोनों ही एक-दूसरे के स्नेह के लिए तरसते हैं। पुत्र माँ से फोन पर बात करता है तब पुत्र की आवाज सुनकर ही माँ की ममता आँसुओं के रूप में फूट पड़ती है। माँ को रोते देखकर पुत्र को लगता है माँ बे-वजह ही रोती है। परंतु जब फोन पर बातचीत पूरी हो जाती है और पुत्र फोन रखता है तो वह भी अपनी भावनाएँ रोक नहीं पाता और रोता है।
पिता माँ की अपेक्षा कठोर हृदय के होते हैं ऐसा लोग कहते हैं, परंतु उनका हृदय भी कोमल ही होता है। जब संतान को चोट लगती है तो वह तड़प उठते हैं। यह इस बात का सबूत है कि पिता में भी कोई माँ छुपी होती है अर्थात स्नेह की बात आती है तो माँ के ममत्व (mother’s love) को अधिक अहमियत दी जाती है परंतु पिता भी अपनी संतान से उतना ही स्नेह करते हैं जितना कि एक माँ।
![]()
जीवन की जरूरतें पूरी करने हेतु माता-पिता दोनों काम पर जाते हैं। कभी-कभी शिफ्ट में ड्युटी करने वाले अभिभावक (guardian) अपनी ड्युटी इस तरह से लेने की कोशिश करते कि दोनों में से एक बच्चे के साथ रहे। ऐसे में पिता महीनों तक दिन की शिफ्ट जाता है और माता महीनों तक रात की शिफ्ट जाती है। बच्चे को दोनों का स्नेह ऐसी स्थिति में टुकड़ों में ही मिलता है। नन्हा बच्चा जब माँ का स्नेह पाता है तब पिता नहीं होते और पिता का स्नेह पाता है तब माँ काम पर गई होती है।
उगते सूरज का स्वागत सभी करते हैं, परंतु डूबते सूरज के प्रति उदासीनता दिखलाते हैं। कवि का मशवरा है कि डूबते सूरज को भी नहीं भूलना चाहिए क्योंकि, डूबना-उगना तो केवल नजरों का धोखा है। सूरज तो आसमान में ही है। हमारी धरती ही सूरज की परिक्रमा करते हुए हमें सूरज के डूबने-उगने का आभास कराती है अर्थात सच्चाई से मुख न मोड़कर हमें हर स्थिति में तटस्थ रहना चाहिए।
जीवन पथ पर चलते-चलते कभी-कभी गिर भी गए तो खुद को सँभालकर आगे बढ़ना चाहिए। गिरने पर जो चोट लगती है वह हमें जीवन का नया पाठ सिखाती है क्योंकि ठोकर खाकर ही हमें जीने की कला का सबक मिलता है। अनुभव से बड़ा कोई शिक्षक नहीं। बस, जीवन चलने का नाम है, आगे बढ़ने का नाम है इसे याद रखना होगा।
जीवन पथ पर आगे बढ़ते समय कभी मार्ग में घनघोर अँधकार छाया हुआ मिले लेकिन तब भी हमें उम्मीद नहीं छोड़नी चाहिए। किसी दिन उजाला अवश्य होगा यही आशा रखते हुए आगे बढ़ना चाहिए क्योंकि रात की कोख से ही सुबह जन्म लेती है। इस त्रिवेणी में कवि हमें निराशा के अंधकार से आशा के उजाले की ओर ले जाना चाहते हैं और इस सत्य से अवगत (aware) कराते हैं कि जीवन में दुःख के बाद सुख अवश्य आता है। सुख-दुख, दिन-रात की तरह आते-जाते रहते हैं इसीलिए दुख की स्थिति में निराश नहीं होना चाहिए।
अपनी आयु के कुछ पल उधार लेकर मैंने हसरत के बीज बोए क्योंकि विचारों की जमीन मेरे पास पहले से थी अर्थात (means) हमारे सपनों को पूरा करने के लिए आयु कम ही पड़ती है। परंतु सपने अवश्य देखने चाहिए और उन्हें पूरा करने की कोशिश भी करनी चाहिए।
मंजिल बहुत दूर है यह सोचकर हमें यात्रा रोकनी नहीं चाहिए बल्कि छोटे से दीपक की धुंधली सी रोशनी में भी यात्रा आरंभ कर लेनी चाहिए क्योंकि हिम्मत से आगे बढ़ने वाले यात्री के कदम जहाँ भी पड़ेंगे वहाँ रोशनी होगी और मंजिल का मार्ग प्रशस्त (wide) होगा। कवि हमें सकारात्मक सोच से जीवन में आगे बढ़ने का मशवरा दे रहे हैं।
![]()
मैंने जब कभी अपनी आँखों में देखा स्वयं को एक बालक की तरह अबोध, (innocent) निष्पाप पाया। इससे तो यही सिद्ध होता है कि उम्र बढ़ती ही नहीं। वास्तव में उम्र बढ़ती है वैसे-वैसे हम मासुमियत खो देते हैं और दुनियादारी निभाते-निभाते हमारे भीतर छिपे बालक को बड़ा बुजुर्ग, अनुभवी बनाकर जीवन की सुंदरता का गला घोट देते हैं। मन को अशांत, तनाव पूर्ण, कुंठा (frustration) ग्रस्त बना देते हैं। इससे मुक्ति पाना है तो खुद के भीतर छिपे बालक को जीवित रखना चाहिए।
आँसू और खुशियाँ एक ही वस्तु के दो नाम हैं। जैसे पेड़ में बीज छुपा है और बीज में पेड़ वैसे ही आँसू में खुशी और खुशी में आँसू छिपे होते हैं। जो इस बात को समझ गया वही सच्चा ज्ञानी है क्योंकि आँसू और खुशियाँ जीवन के दो अंग हैं और ये जीवन में आते-जाते रहते हैं। हर खुशी में आँसू छिपे हैं जो हमारी भावनाओं के प्रतीक (symbol) हैं और आँसुओं के जल से सींचकर खुशियों का वृक्ष फलता-फूलता है।
जीवन में कितनी ही कठिनाइयाँ क्यों न आए हमें उम्मीद का दामन कभी नहीं छोड़ना चाहिए। क्योंकि नाउम्मीदी तो मृत्यु के समान है अर्थात् कठिनाइयों से घबराकर निराशा का दामन थामने वाला जीते जी मर जाता है। जैसे हर अँधेरी रात के बाद सुबह अवश्य आती है वैसे ही दुख के बाद सुख का आना निश्चित है। इस सकारात्मकता (positivity) के साथ जीना ही जिंदगी है। सुख-दुख की स्थिति में स्थिर रहना ही मनुष्य की सच्ची पहचान है इस तथ्य को कभी नहीं भूलना चाहिए।
प्रेरणा शब्दार्थ:
- घनघोर = घना
- हसरत = चाह, इच्छा
- आगाज-ए-सफर = यात्रा का आरंभ
- शय = वस्तु, चीज
- सख्त = कठोर (strict),
- नाजुक = कोमल (sensitive),
- महज = केवल, मात्र (just),
- चोट = आघात, प्रहार, घाव (hurt),
- घनघोर = घना, डरावना (dense),
- आस = आशा, भरोसा (hope),
- कोख = पेट, गर्भाशय (womb),
- लम्हा = पल, क्षण (moment),
- हसरत = चाह, इच्छा (desire),
- खयाल = स्मृति, विचार (idea),
- आगाज-ए-सफर = यात्रा का आरंभ (set off),
- शय = वस्तु, चीज (thing),

- दामन = आँचल, पल्लु (fringe),
- जरा = थोड़ा सा (little bit)
Hindi Yuvakbharati 11th Digest Maharashtra Board
- प्रेरणा Question Answer
- लघु कथाएँ Question Answer
- पंद्रह अगस्त Question Answer
- मेरा भला करने वालों से बचाएँ Question Answer
- मध्ययुगीन काव्य (अ) भक्ति महिमा Question Answer
- मध्ययुगीन काव्य (आ) बाल लीला Question Answer
- कलम का सिपाही Question Answer
- स्वागत है ! Question Answer
- तत्सत Question Answer
- गजलें Question Answer