Marketing 12th OCM Chapter 8 Solutions Maharashtra Board
Balbharti Maharashtra State Board Organisation of Commerce and Management 12th Textbook Solutions Chapter 8 Marketing Textbook Exercise Questions and Answers.
Class 12 OCM Chapter 8 Exercise Solutions
1. (A) Select the correct options and rewrite the sentence
Question 1.
The term market is derived from the ………………. word ‘mercatus’.
(a) French
(b) Latin
(c) Italian
Answer:
Latin
Question 2.
In the ………………. concept of market, emphasis is given on ‘buying and selling of goods or services’.
[a) Place
(b) Exchange
(c) Customer
Answer:
Exchange

Question 3.
Perishable goods such as vegetables, fruits, milk products, etc. are sold in ………………. Market
(a) Very Short Period
(b) Short Period
(c) Long Period
Answer:
Very Short Period
Question 4.
Retail market is the market where retailer sells goods directly to the ……………… in small quantities.
(a) producer
(b) wholesaler
(c) consumer
Answer:
consumer
1. (B) Match the pairs
Question 1.
| Group A |
Group B |
| (A) Market |
(1) Single Seller |
| (B) Registered brands |
(2) Stock Market |
| (C) Monopoly |
(3) Distinct Name |
| (D) Branding |
(4) Mercatus |
| (E) Digital |
(5) Single Buyer |
|
(6) ISI |
|
(7) Trademark |
|
(8) Use of traditional media Marketing |
|
(9) Multiple seller |
|
(10) Use of digital media |
Answer:
| Group A |
Group B |
| (A) Market |
(4) Mercatus |
| (B) Registered brands |
(7) Trademark |
| (C) Monopoly |
(1) Single Seller |
| (D) Branding |
(3) Distinct Name |
| (E) Digital |
(10) Use of digital media |
1. (C) Give one word/phrase/term for the following statements
Question 1.
The market for the commodities which Eire produced in one country and sold in other countries.
Answer:
International market
Question 2.
Type of market where durable commodities which are generally non-perishable in nature are sold.
Answer:
Long period Market
Question 3.
The market where goods Eire sold to the ultimate consumers or the users of the product.
Answer:
Retail Market
Question 4.
Two sellers, selling either a homogeneous product or a differentiated product.
Answer:
Duopoly
Question 5.
Giving of distinct name to one’s product.
Answer:
Branding
1. (D) State whether following the statements are True or False
Question 1.
The term market is derived from the Latin word ‘mercatus’.
Answer:
True
Question 2.
Market may be defined as aggregate demand by potential buyers for a product or service.
Answer:
True
Question 3.
The market for the commodities which are produced in one country and sold in another countries is known as national market.
Answer:
False

Question 4.
In wholesale market sellers are known as retailers and buyers are known as wholesaler.
Answer:
False
Question 5.
Regulated Market operates according to forces of demand and supply.
Answer:
False
1. (E) Find the odd one
Question 1.
Primary Market, Secondary Market, Retail Market, Terminal Market
Answer:
Retail Market
Question 2.
Stock Exchange, Foreign Exchange, Bullion Market, Manufactured Goods Market
Answer:
Manufactured Goods Market
Question 3.
Price, People, Promotion, Product
Answer:
People
Question 4.
People, Promotion, Physical Environment, Process
Answer:
Promotion
1. (F) Complete the sentences
Question 1.
……………. is the place where transaction of buying and selling of goods and services take place in exchange of money or money’s worth.
Answer:
Market
Question 2.
…………… refers to a market situation when there is a single buyer of a commodity or service.
Answer:
Monopsony
Question 3.
…………….. is a slip which is found on the product and provides all the information regarding the product and its producer.
Answer:
Label
1. (G) Select the correct option and complete the following table
(Mercatus, Industrial goods, Oligopoly, E. Jerome McCarthy, Booms & Bitner)
|
Group A |
Group B |
| 1. |
Commodity Market |
————– |
| 2. |
————– |
A few sellers |
| 3. |
—————— |
To trade merchandise |
| 4. |
4 Ps |
————– |
| 5. |
————– |
3 Ps |
Answer:
|
Group A |
Group B |
| 1. |
Commodity Market |
Industrial goods |
| 2. |
Oligopoly |
A few sellers |
| 3. |
Mercatus |
To trade merchandise |
| 4. |
4 Ps |
E Jerome McCarthy |
| 5. |
Booms & Bitner |
3 Ps |
1. (H) Answer in one sentence
Question 1.
What do you mean by ‘Local Market’?
Answer:
Local Market refers to local geographical area in the region within which goods and services are bought and sold, e.g. purchase of grocery from the grocery shop located near to residential area.
Question 2.
What do you mean by ‘Regulated Market’ ?
Answer:
Market which is governed (regulated) by the statutory or legal provisions of the country is called regulated market, e.g. Stock exchange, Foreign exchange, etc.
Question 3.
Define Market.
Answer:
Market is the place where two or more parties, i.e buyer and seller are involved in buying and selling of goods and services for money or money’s worth.
Question 4.
What do you mean by Standardisation?
Answer:
Standardisation means setting up of standards or fixing certain norms in relation to design, quality, size, process, weight, colour, etc. of the product.
Question 5.
What do you mean by Branding?
Answer:
Branding refers to a process in which separate identity to a product is given through unique brand name to differentiate it from other products.
1. (I) Correct the underlined word and rewrite the sentence
Question 1.
Commodity Market refers to the market for borrowing and lending long term capital required by business.
Answer:
Capital Market refers to the market for borrowing and lending long term capital required by business.
Question 2.
In duopoly there is a single seller.
Answer:
In monopoly there is a single seller.

Question 3.
Pricing bridges the time gap between production and sale of goods.
Answer:
Warehousing bridges the time gap between production and sale of goods.
Question 4.
Branding is the process of classification of products according to similar characteristics and/or quality.
Answer:
Grading is the process of classification of products according to similar characteristics and/or quality.
Question 5.
Grading helps in avoiding breakage, damage and destruction of the product.
Answer:
Packaging helps in avoiding breakage, damage and destruction of the product.
1. (J) Arrange in proper order
Question 1.
Local market, International market, National market.
Answer:
Local market, National market, International market.
Question 2.
Grading, Market Planning, Distribution.
Answer:
Market Planning, Grading, Distribution.
2. Explain the following terms/concepts
Question 1.
Market.
Answer:
(1) The word ‘market’ has originated from the Latin word ‘mercatus’ which means ‘to trade’, ‘to trade merchandise’ or ‘a place where business is transacted’. Thus, market refers to the specific place where buyers, sellers and other middlemen gather and buy and sell commodities for certain price which is predetermined or bargained.
(2) In earlier days, place played a significant role in defining the term ‘market’. However, in modern days of information technology it has got wider meaning. Now the term ‘market’ is no more restricted to specific place or area, but it implies a complex network of dealers linked physically by telephone, computer, internet and many other arrangements and facilities which effect transfer of goods and services at a place.
Question 2.
Place Concept of Market.
Answer:
Place concept of market : The place concept of market is usually taken to mean the place where the goods and services are bought and sold for money or money’s worth. In the place concept of market, emphasis is given on the place where trading takes place. It is referred to a place where buyers, sellers and other intermediaries meet and buy and sell goods and services. In ancient days the term market was defined on the basis of place only. However in recent era of information and technology market has wider meaning and not just a place.
Question 3.
Commodity Concept of Market.
Answer:
Commodity concept of market : In this concept of market more stress (significance) is given on exchange of goods and services. In this concept the process of exchange of goods and services is more important and not the place of exchange. The buyer and seller and commodity bought and sold among them play significant role in this process. Accordingly, commodity concept of market means ‘buying and selling of goods and services’.
Question 4.
Digital Concept of Market.
Answer:
Space or Digital concept of market : Growth and development of information technology gave rise to the new concept of the market known as space or digital concept. Now people can buy and sell any commodity easily and conveniently with the help of new and sophisticated E-commerce portals and mobile applications. The direct contact between buyer (customer) and seller is now possible through the communication media such as telephone, mobile, computer, internet, etc.
Question 5.
Product.
Answer:
Product : An article, goods, commodity, or service that is manufactured and offered to the customers for sale is called product. It has capacity to satisfy desire or need of the customers. The products may be either tangible or intangible. It can be in the form of goods or services. By undertaking extensive market research the business enterprises are required to decide the right type of products to be produced and sold. The product creates impact on the mind of the customers on which success or failure of business firm depends.
Question 6.
Price.
Answer:
Price : The amount of money given or required to buy a product is called price. It is essentially the amount that customer ready to pay for a product. The cost of the product, willingness of the customers to pay for the product, value and utility of the product are the main factors that businessmen have to considered while (determining) deciding the price of a product. Price of the product should be neither too high which affects the demand adversely nor too low which reduces the profitability of the business.
Question 7.
Promotion.
Answer:
Promotion : Promotion is one of the basic elements of marketing because it makes the customers aware of its product, creates brand recognition and sales. Promotion refers to any type of marketing communication used to inform and persuade potential buyers or consumers to buy the products by explaining them the regular merits of products, service, brand, etc. Promotion mix comprises of different tools such as advertising, sales promotion, direct marketing, personal selling, etc. promotional strategies to be used in the business depends on various factors such as budget, target market and the message wants to communicate.

Question 8.
Marketing Mix.
Answer:
(1) The term ‘marketing mix’ is of recent origin and it is often used in modern marketing. Marketing mix refers to the mixture or combination of various marketing variables that the business enterprises intermix and control to get expected result from the target market. In other words, marketing mix means placing the right product, at right price, in right place and at right time. It is considered as one of the important tools of marketing. Every business enterprise must develop appropriate marketing mix to expand turnover and achieve its objectives.
(2) The 4 Ps of marketing mix viz. product, price, place and promotion were introduced by E. Jerome McCarthy in 1960. Then in 1981, 4 Ps were further extended by Booms and Bitner by adding 3 new elements viz. People, Process and Physical Environment. The marketing mix is broadly categorised into two types, viz. Product Marketing Mix (first 4 Ps) and Service Marketing Mix (newly added 3 Ps). In brief, marketing mix implies taking appropriate decisions in the above stated 7 broad areas which are supplementary to one another by the business enterprise.
Question 9.
Packaging.
Answer:
Packaging : The term ‘Pack’ means a collected amount of anything wrapped up or tied together for carrying. Package is a parcel, box or container in which things are packed accordingly, packaging means a process by which a finished product is made ready for storage, delivery or transportation, usually by placing the product in container or providing it with a wrapping. It implies designing the package for the product. The aim of packaging is to protect the goods during transport and storage and to preserve the contents and the quality of the product. It facilitates easy handling of goods in transit and helps to avoid breakage, leakage, damage, spoilage, pilferage and destruction of the product.
Question 10.
Labelling.
Answer:
Labelling : The term, ‘label’ means a piece of paper, card or other material attached to an object. It is a slip affixed on the product which provides all detail information in respect to product and its producers. It also gives details of ingredients and their quantity used in the product. Labelling also gives information about the product i.e. its weight, size, price, date of manufacture and expiry name and address of manufacturer, etc. The slip on which all these information are printed is called label and its process is called labelling. A label may be part of the package or it may be attached as a tag on the product. Labelling serves as an effective tool of marketing the product. Label is used to indicate the brand, grade, quality, etc. of the product. Good labelling adds to products overall attractiveness.
3. Study the following case/situation and express your opinion
Question 1.
Mr X purchases goods from nearest shop. Mr Y purchases mobiles from Tokyo. Mr Z of Nandurbar purchases electronic goods from Delhi.
(i) From which type of market does Mr. X purchase?
(ii) Name the type of market from where Mr. Y deals.
(iii) State the type of market from where Mr. Z purchases goods.
Answer:
(i) Mr. X purchases goods from Local market.
(ii) Mr. Y purchase mobiles from Tokyo (foreign market) and hence he deals in International market.
(iii) Mr. Z of Nandurbar purchases electronic goods from Delhi, i.e. from National market.
Question 2.
Mr. X deals in import and export business so he needs different foreign currencies. For the expansion of his business, he borrows money from bank. He invests his funds in the equity shares.
(i) Name the market from where Mr. X borrows money.
(ii) Name the market where Mr. X invests his funds.
(iii) Which type of currency is required for international market?
Answer:
(i) Mr. X borrows money from capital market.
(ii) Mr. X invests his funds in the regulated market.
(iii) The different foreign currency is required by Mr. X for international market.
4. Answer in brief
Question 1.
Explain in detail the significance of marketing to the society.
Answer:
(1) Increase in standard of living : The main aim of marketing is to supply goods and services to fulfil the customers’ requirements. Marketing finds out the requirements of the customers and accordingly make efforts to supply quality products at cheaper prices. This in turn helps to maintain and raise the standard of living of the customers. In recent era, large scale production of products and services have considerably reduced their prices due to which poor people also attain reasonable level of living.
(2) Provide employment : Modern marketing i undertakes almost all functions of organisation such as buying, selling, financing, transport, warehousing, risk bearing, research and development, etc. which generate and create more job opportunities in different capacities and help to solve the problem of unemployment.
(3) Decreases distribution coats : Effective and proper utilisation of channel of distribution reduces overall cost of the products and services, Thus, marketing activities make the goods and services available to the society at cheaper prices. Due to reduction in the cost, number of potential consumers will also increase. It also gives guarantee of timely availability of the product.
(4) Consumer awareness : Marketing helps the society by educating consumers and by giving information of new products and services available in the requirements of consumers by providing relevant information, goods and services to the people as per their demand and taste. Marketing also includes information to help in making a purchase.
(5) Increase in National income : The scientifically well organised marketing activities facilitates rapid economic development of the nation. Well organised and effective marketing of products and services facilitates industrialisation, increases job opportunities and develop the economy. Effective marketing integrates agriculture and industry which in turn bring rapid development in the country.
(6) Managing consumer expectations : Marketing research enables the business organisations to understand the requirements of the consumers which helps in development of products to satisfy the customers expectation. The business organisations make certain changes in the product by considering customers’ review. Through marketing, business enterprises inform consumers about the major changes such as mergers, transfer in ownership which affect product offerings. Government regulations stop marketers to make false and misleading claims.
Question 2.
Explain 4Ps of product marketing mix.
Answer:
7 Ps of marketing are explained as follows:
(1) Product : An article, goods, commodity, or service that is manufactured and offered to the customers for sale is called product. It has capacity to satisfy desire or need of the customers. The products may be either tangible or intangible. It can be in the form of goods or services. By undertaking extensive market research the business enterprises are required to decide the right type of products to be produced and sold. The product creates impact on the mind of the customers on which success or failure of business firm depends.
(2) Price : The amount of money given or required to buy a product is called price. It is essentially the amount that customer ready to pay for a product. The cost of the product, willingness of the customers to pay for the product, value and utility of the product are the main factors that businessmen have to considered while (determining) deciding the price of a product. Price of the product should be neither too high which affects the demand adversely nor too low which reduces the profitability of the business.
(3) Place : The distribution or placement of product is a significant aspect of the marketing, Place is the element of marketing mix that ensures that right product is distribute and made conveniently available to the potential consumers or buyers at right price, at right location and at right time too. The business enterprises are required to distribute the products at a place easily approachable to the potential consumers or buyers. Place in this respect covers location, distribution and the ways of giving the products in the hand of the customers. The system of distribution decides the coverage of the product in the market.
(4) Promotion : Promotion is one of the basic elements of marketing because it makes the customers aware of its product, creates brand recognition and sales. Promotion refers to any type of marketing communication used to inform and persuade potential buyers or consumers to buy the products by explaining them the regular merits of products, service, brand, etc. Promotion mix comprises of different tools such as advertising, sales promotion, direct marketing, personal selling, etc. promotional strategies to be used in the business depends on various factors such as budget, target market and the message wants to communicate.

Question 3.
Explain 3Ps in marketing of services.
Answer:
1. People : The employees, i.e. people inside the business and consumers/customers, i.e. people outside of the business create direct or indirect impact (influence) on the business. People include all individuals that play key role in offering the product or service to the buyer or customer. People inside the business comprise employees who assist to deliver services to the customers. Right people appointed to work at right place definitely add value to the business. The management or employer of the business enterprises are required to recruit right people, train them, develop their skill and retain them for their success.
2. Process : The steps taken by the business organisation in carrying the products and services from the place of business to the doorsteps of customer are called process. Processes are significant to provide a quality service. Good process ensures same standard of service to the customers, save time and cost by enhancing efficiency. The growth and development of technology enable the business organisations in effective monitoring of the process of business and help to take corrective action if necessary.
3. Physical environment : Marketing environment in which the interaction between customer and firm takes place is called physical environment. While providing (offering) intangible services, the service providers always try to incorporate certain tangible elements into their offering to increase experience of customers. In the service market, physical or tangible evidence is essential to ensure that the service is successfully provided. Physical evidence enables the customers to trace conveniently the brand leaders in the market. The physical evidence comprises of location, layout, packaging, branding, interior design, dress of the employees, their action, waiting area, etc.
Question 4.
Explain types of market on the basis of area covered.
Answer:
On the basis of area covered:
(i) Local market: Local market refers to local geographical area in the region within which goods and services are bought and sold, e.g. purchase of grocery from grocery shop located in a market near to residential place.
(ii) National market : National market refers to the area of a country within which goods and services are bought and sold, e.g. purchase of apples on wholesale from the state of Haryana by a customer staying in Mumbai.
(iii) International market : The market where the goods and services which are manufactured in one country and sold in many other countries is called international market, e.g. purchase of petrol by Government of India from Saudi Arabia.
Question 5.
Explain types of market on the basis of time.
Answer:
On the Basis of time:
(i) Very short period market : The market which is organised and carried on for very short period of time is called very short period market. This market has existence for very short period say few hours or for a day at a particular time and place, e.g. market for perishable goods such as vegetables, milk, fish, milk products, meat, fruits, etc.
(ii) Short period market : The market which is organised and carried out its functions for short period of time is called short period market. This market has existence for a short period, weekly market, festival market, market during fairs, etc. are known as short period market. In this market mostly perishable goods and semi-durable goods are bought and sold.
(iii) Long period market : The market which is organised and continued its functions for long period of time is called long period market. This market has existence for long period of time. In this market usually durable commodities are bought and sold.
5. Justify the following statements
Question 1.
Marketing is significant to the consumers.
Answer:
Importance of marketing to the consumers:
(1) Promotes product awareness : Many companies undertake different marketing activities to promote their products and services. Marketing makes consumers aware about the different products, various brands and features of products and services available in the market. Marketing facilitates the consumers to take right decision on purchases of products. On the basis of marketing information consumers can compare product features, availability, price and other essentials. Marketing also improves the quality of life of the consumers.
(2) Provides quality products : On account of increasing competition in the market, consumers easily get information about the product and services available in the market. Marketing creates moral pressure on the business enterprises to sell quality products and services to the consumers. If business enterprises supply defective products to the consumers, sooner or later, it will create negative image of the business and adversely affect customers’ loyalty towards business and products.
(3) Provides variety of products : Marketing provides information to the consumers about the product and attracts them to purchase the same market segment or marketing plan becoming critical in any business on account of increase in customer population their preferences and availability of competitive options. The business organisation are required to launch product by considering market segment. Business organisations are required to make available variety of goods to fulfil the requirements of different market segments of the consumers. According to price, size and quality of products, variety’ may change.
(4) Helps in selection : In the competitive markets, different variety of products with different brands are available. Marketing enables the consumers to select the best products and services from the different options available.

Question 2.
Promotion plays an important role in marketing.
Answer:
(1) Promotion means any type of marketing communication used by the business enterprises to inform and persuade potential buyers to buy the products or services. In promotion merits of products, services, brand benefits, etc. are explained to the potential buyers to attract them towards the products. Promotion mix comprises of different tools such as advertising, sale, promotion, direct marketing, personal selling, publicity, etc.
(2) Business enterprises make use of combination of all or some of these five methods for promotion of their products and services as per the need of the business. Promotional activities help the organisation to increase brand awareness in the market. In brief promotion is concerned with informing the customers about the products of the firm and persuading them to buy the products.
(3) Promotional strategy to be used in the organisation depends on the various factors such as budget, the message it wants to communicate and target market. Promotion is an important element of marketing that creates brand recognition and ultimately increase sales.
(4) Business enterprises through promotion attract consumers to buy and taste the products. Promotion mix to be used will depend upon the nature of the product, type of customers, stage of demand, degree of competition, etc. Promotion helps business organisations to enhance the sales. Increase in sales, increases profit for the organisation. If the increased profit and income is reinvested in the business, it will earn more and more profits in the future. Promotion helps the organisation to create awareness about existing products, new arrivals and the company selling certain products in the market. It creates brand image among the buyers. Business organisations also use promotion to build customers’ layout base. This in turn expands sales and profits.
Question 3.
Market can be classified on the basis of competition.
Answer:
On the basis of competition:
(i) Perfect market : A type of market in which large numbers of buyers and large numbers of sellers exist to buy and sell homogeneous product at prevailing market price is called perfect market. In this market, all buyers and all sellers have equal access to all information and have perfect knowledge about the market conditions. Neither single buyer nor a single seller can influence price. One uniform single price prevails in the market, This model of perfect market is frequently used in economic analysis.
(ii) Imperfect market : A type of market which has distinct features of market imperfection such as single seller, imperfect knowledge of market conditions on the part of buyers or sellers, failure to make adjustment in demand and supply, etc. is called imperfect market, e.g. monopoly market. Imperfect market is further sub-divided into the following categories:
(a) Monopoly : The word monopoly is the combination of two words, viz. ‘mono’ means one and ‘poly’ means seller. Accordingly, a market structure which is characterised by a single seller selling unique product in the market is called monopoly. In monopoly, seller faces no competition as he is the sole seller of goods with no close substitute. He controls the entire supply and enjoys the power of setting the price for his goods. He is price maker.
(b) Duopoly : A market situation in which two suppliers dominate the entire market for a commodity or service. In this market, there are two sellers, who either sell a homogeneous product or differentiated product. They enjoy a monopoly in the product produced and sold by them.
(c) Oligopoly : Oligopoly is a state of limited competition in which market is shared by a limited number (few) of producers or sellers producing and selling either a homogeneous product or a differentiated product.
(d) Monopsony : Market situation in which there is only one buyer substantially controls the market as major and single purchaser of goods and services offered by many producers or sellers, is called monopsony market, e.g. labour market, a firm is the sole purchaser of a certain kind of labour.
Question 4.
Marketing helps in increasing consumer awareness.
Answer:
(1) Many companies undertake different marketing activities to promote their products and services. Marketing makes consumers aware about the different products, brands, features and usefulness of the products and services available in the market. Marketing facilitates the consumers to take right decisions on the purchases of right products and services.
(2) Marketing helps the society by educating consumers and through giving information of new products and services available in the market and their usefulness. Marketing identifies and satisfies the requirements of consumers by providing relevant information, goods and services to the people as per their demand and preferences. Marketing provides information to consumers to take right decisions.
(3) On the basis of marketing information, consumers can compare products features, availability, price and other essentials and chooses right products at right prices. Marketing also helps to improve the quality of life of the consumers. Marketing helps to create awareness about the existing products, new products entering into the market and the company selling specific products in the market. This in turn helps to raise awareness among the potential and prospective consumers. Marketing also creates brand image among the consumers.
(4) The business organisations use marketing to create awareness among the consumers regarding major changes such as mergers and transfer of ownership that influence products offerings or way to improve quality. Thus, marketing creates awareness among the consumers about the products available in the market and attracts the customers to buy the same.

Question 5.
There is a need of branding to get’ recognition among the consumers.
Answer:
(1) A name, design, term, symbol, sign, numeral or combination of them given to the product to identify the goods or services of the one manufacturer and to differentiate them from those of competitors, is called a brand. Branding, therefore means, giving a distinctive identity to a product. Bata, Surf, Coca-Cola, 501 soap, 50-50, etc. are some of the well-known brands. Registered brand is called trade mark. Trade mark cannot be copied by other firms. Branding is done mostly for manufactured goods.
(2) Brands are very effective for wide publicity. It helps sampling. Effectively established brands increases value of business in the industry. Good brand expands the business and create a positive impact on the business and the customers. The customers attracted towards the products are assured dependability and loyalty by using branded products. Strong branding helps to generate new customers and maintain the existing and regular customers.
(3) Branding helps the products and producers to get recognition among the consumers. Branding facilitates advertising and price control. Branded goods enjoy a wider market as the necessity or personal inspection or sample is avoided. By registering his brand, a businessman can protect his products from imitation.
(4) Branding helps to get new business and increase brand awareness in the market. It creates trust in the mind of consumers, potential consumers and people. The consumers prefer to do business with a company that has professional brand name in the market.
6. Attempt the following
Question 1.
Give classification of market in detail on the basis of ‘Volume of Transaction
Answer:
On the basis of volume of transaction:
(i) Wholesale market : Wholesale market refers to market in which goods purchased and sold in bulk (large) quantity at lower (cheaper) prices. In this market goods are purchased by the wholesalers in large quantity from manufacturers. He then sells these goods in moderate quantity to retailers who then sell these goods to consumers in small quantity. In brief, it is a market for bulk purchases and sales of goods. In this type of market, wholesalers are know as sellers and retailers are known as buyers.
(ii) Retail market : Retail market refers to market in which retailers sell goods directly to the ultimate consumers in small quantity or one to one basis, e.g. General provisional goods shop localised in market.
Question 2.
Give classification of market on the basis of ‘Time’.
Answer:
On the Basis of time:
(i) Very short period market : The market which is organised and carried on for very short period of time is called very short period market. This market has existence for very short period say few hours or for a day at a particular time and place, e.g. market for perishable goods such as vegetables, milk, fish, milk products, meat, fruits, etc.
(ii) Short period market : The market which is organised and carried out its functions for short period of time is called short period market. This market has existence for a short period, weekly market, festival market, market during fairs, etc. are known as short period market. In this market mostly perishable goods and semi-durable goods are bought and sold.
(iii) Long period market : The market which is organised and continued its functions for long period of time is called long period market. This market has existence for long period of time. In this market usually durable commodities are bought and sold.
Question 3.
Explain packaging and labelling.
Answer:
(1) Packaging : The term ‘Pack’ means a collected amount of anything wrapped up or tied together for carrying. Package is a parcel, box or container in which things are packed accordingly, packaging means a process by which a finished product is made ready for storage, delivery or transportation, usually by placing the product in container or providing it with a wrapping. It implies designing the package for the product. The aim of packaging is to protect the goods during transport and storage and to preserve the contents and the quality of the product. It facilitates easy handling of goods in transit and helps to avoid breakage, leakage, damage, spoilage, pilferage and destruction of the product.
Sealed packaging reduces the chances of adulteration or duplication of the products. Materials like bottles, containers, plastic bags, bubble bags, tin, jute bags, wooden boxes, packing foam, etc. are used for packing the product. Packaging decorates and beautifies a product and thereby creates a desire in the minds of the customers to buy the product even through they have no immediate need for it. Good packing increases durability of the product. In brief, packaging is an indispensable technique by which the goods are protected, handled, identified, advertised and sold on large scale. Thus, modern packaging promotes sales.
(2) Labelling : The term, ‘label’ means a piece of paper, card or other material attached to an object. It is a slip affixed on the product which provides all detail information in respect to product and its producers. It also gives details of ingredients and their quantity used in the product. Labelling also gives information about the product i.e. its weight, size, price, date of manufacture and expiry name and address of manufacturer, etc. The slip on which all these information are printed is called label and its process is called labelling. A label may be part of the package or it may be attached as a tag on the product. Labelling serves as an effective tool of marketing the product. Label is used to indicate the brand, grade, quality, etc. of the product. Good labelling adds to products overall attractiveness.
Question 4.
Explain standardisation and grading.
Answer:
(1) Standardisation : The term standard means an object, quality or measure serving as a basis or example to which others should confirm. It is a criterion, rule norm or measurement used for distinguishing one product from another so far its quality is concerned. Accordingly standardisation means fixing or determining certain norms or criteria with regard to the quality of a product. It may be related to process, size, quality, design, weight, colour, etc. of the product. It is a mental process of establishing standard as indicator of certain quality. It facilitates grading. Standardised commodities are easily and quickly sold even at high price in the market due to uniformity in their quality. Standardisation helps to achieve customers’ loyalty towards the product.
(2) Grading : The term ‘grade’ means a class or a category. Accordingly grading means physical sorting or classifying of products into different categories of similar characteristics or quality or set standards. Grading is done on the basis of certain features like size, shape, quality, etc. On the basis of grading, the goods are described as superior, good, better, best, inferior, medium, etc. It is necessary in agriculture, dairy, forest and mineral products. Standardisation and grading are interrelated process because without standardisation grading cannot take place and standardisation has no scope without grading.

Question 5.
Explain importance of marketing to the firm.
Answer:
The importance of marketing to the firm is explained as follows:
(1) Increases awareness : Marketing provides information and creates understanding among the consumers about the existing products, new arrivals and the company selling a particular product in the market. This in turn increases awareness among the potential consumers and creates brand image among the consumers.
(2) Increases sales : After providing information successfully about the products or services among the consumers, marketing attracts them to buy the products or services. Successful marketing campaign enables the business organisations to enhance the sales of the organisation. Expansion in sales increases profit for the organisation which is in turn reinvested in the business to earn more and more profits in the future. In recent era, survival of the business organisations depends on the effective and efficient marketing function.
(3) Create trust : The consumers usually prefer to buy required goods and services from those business enterprises which have trustworthy reputation in the market. To create trust among the customers longer time is required. Trustworthy business enterprises earn loyal customers. This trust worthiness creates customers loyalty. Satisfied customers enhance the brand image of the organisation in the market. Efficient and effective marketing plays significant role in creating good relationship between the customers and the organisation. Efficient pricing policy and appropriate after sales service improve image of the business enterprises. Majority of marketing activities move towards building brand equity of the business.
(4) Basis of making decisions : Every business organisation is required to take several decisions before delivering the final products or services to the consumers. The business organisation is required to considered or face many problems such as what to produce, how to produce, when to produce, and for whom to produce? When business expands, the decision making process becomes more complex. Effective marketing facilitates organisation to take right decisions at right time.
(5) Source of new ideas : Marketing enables the business organisations to know the requirements of the consumers. Feedbacks received from the consumers helps the manufacturers or producers to make improvement or desired changes in the existing products. Tastes and preferences of the consumers change rapidly. Due to marketing, business enterprises understand these changes and new demand pattern emerges in the market. Accordingly, Research and Development department develops the products. In the field of product development 4 Ps of marketing mix viz. product, price, place and promotion play major role. Inventions and innovations are made by the Research and Development team of the business when the need arises.
(6) Tackling the competition : On account of ; increasing competition among all the sectors of the economy, it is now difficult for any business enterprise to create monopoly for its products or services. Marketing creates brand loyalty in the mind of potential buyers. Marketing facilitates organisations to communicate the salient features and advantages of products and services to consumers and induce them to buy the same. Efficient marketing strategies depict better image of the business than competitors. The efficient management always uses modern technology for effective marketing.
7. Answer the following
Question 1.
Define Marketing and explain in detail the concepts of marketing.
Answer:
[A] Definition : The concept of ‘marketing’ has originated from the term ‘market’. The term ‘market’ implies any arrangement by which the buyers and sellers come into contact directly or indirectly to exchange goods and services at a price. Accordingly, marketing means an action or business of promoting and selling products including market research and advertising. It includes all the activities responsible for the flow of goods and services from the centre of production to ultimate consumers.
In brief, the sum total of all the activities involved in the promotion, distribution and selling of product or service from the producer or seller to the consumer or buyer is called marketing. According to Philip Kotler, “Marketing is a social process by which individuals and groups obtain what they need and want through creating, offering and freely exchanging products and services of value with others’’. Marketing is consumer-oriented. It creates customers for the goods produced in advance.
[B] Concept of marketing : The different concepts of marketing are explained below:
(1) Place concept of market : The place concept of market is usually taken to mean the place where the goods and services are bought and sold for money or money’s worth. In the place concept of market, emphasis is given on the place where trading takes place. It is referred to a place where buyers, sellers and other intermediaries meet and buy and sell goods and services. In ancient days the term market was defined on the basis of place only. However in recent era of information and technology market has wider meaning and not just a place.
(2) Commodity concept of market : In this concept of market more stress (significance) is given on exchange of goods and services. In this concept the process of exchange of goods and services is more important and not the place of exchange. The buyer and seller and commodity bought and sold among them play significant role in this process. Accordingly, commodity concept of market means ‘buying and selling of goods and services’.
(3) Exchange concept of market : Exchange concept of market has given stress on the selling and buying of goods and services between buyers and seller with free consent and mutual trust. During the buying and selling any coercion, undue influence or fraud should not be applied by either party to the transaction. The process between buyer and seller should be voluntary.
(4) Area concept of market: Area concept of market associated with exchange concept. This concept gives more stress on free association between buyers and seller to determine the price of goods to be bought and sold. To fix price it is not essential for buyer and seller to meet each other personally. With the help of different modern communication media, buyers and sellers can fix the price and complete the transaction of exchange of goods or services.
(5) Demand or customer concept of market : According to this concept, customer being the king of the market, so important side of the market is to assess (measure) then needs or demand of the customers. As per this concept market can be studied from the total size of demand or customers. This concept states that the aggregate (total) demand of prospective (potential) buyers for any product is a market.
(6) Space or Digital concept of market : Growth and development of information technology gave rise to the new concept of the market known as space or digital concept. Now people can buy and sell any commodity easily and conveniently with the help of new and sophisticated E-commerce portals and mobile applications. The direct contact between buyer (customer) and seller is now possible through the communication media such as telephone, mobile, computer, internet, etc.
Now it is easy for the customers to get information of quality, features, price, terms and conditions etc. of any products of any company. In brief, Digital market may be defined as, “market which uses Information Technology for buying and selling of the products or services and facilitate communication of quality, features, price, terms of exchange among them.
Question 2.
Explain different types of market in detail.
Answer:
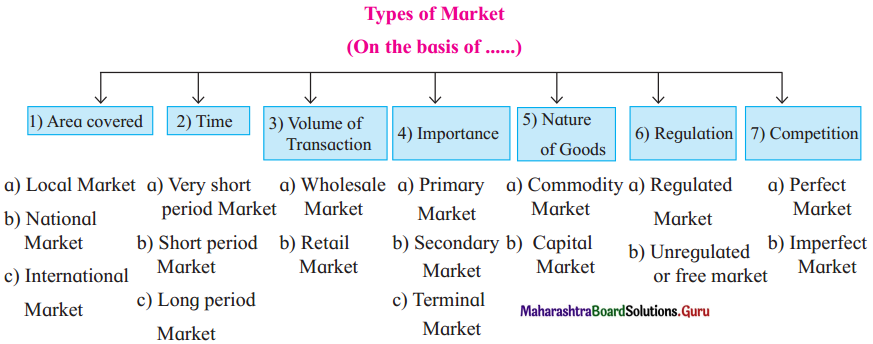
(1) On the basis of area covered:
(i) Local market: Local market refers to local geographical area in the region within which goods and services are bought and sold, e.g. purchase of grocery from grocery shop located in a market near to residential place.
(ii) National market : National market refers to the area of a country within which goods and services are bought and sold, e.g. purchase of apples on wholesale from the state of Haryana by a customer staying in Mumbai.
(iii) International market : The market where the goods and services which are manufactured in one country and sold in many other countries is called international market, e.g. purchase of petrol by Government of India from Saudi Arabia.
(2) On the Basis of time:
(i) Very short period market : The market which is organised and carried on for very short period of time is called very short period market. This market has existence for very short period say few hours or for a day at a particular time and place, e.g. market for perishable goods such as vegetables, milk, fish, milk products, meat, fruits, etc.
(ii) Short period market : The market which is organised and carried out its functions for short period of time is called short period market. This market has existence for a short period, weekly market, festival market, market during fairs, etc. are known as short period market. In this market mostly perishable goods and semi-durable goods are bought and sold.
(iii) Long period market : The market which is organised and continued its functions for long period of time is called long period market. This market has existence for long period of time. In this market usually durable commodities are bought and sold.

(3) On the basis of volume of transaction:
(i) Wholesale market : Wholesale market refers to market in which goods purchased and sold in bulk (large) quantity at lower (cheaper) prices. In this market goods are purchased by the wholesalers in large quantity from manufacturers. He then sells these goods in moderate quantity to retailers who then sell these goods to consumers in small quantity. In brief, it is a market for bulk purchases and sales of goods. In this type of market, wholesalers are know as sellers and retailers are known as buyers.
(ii) Retail market : Retail market refers to market in which retailers sell goods directly to the ultimate consumers in small quantity or one to one basis, e.g. General provisional goods shop localised in market.
(4) On the basis of importance:
(i) Primary market : The market for primary products like agricultural and forest products are called primary market, e.g. market for food grains, vegetables, fruits, etc.
(ii) Secondary market : The market for semi-manufactured goods and semi-processed goods is called secondary market, e.g. iron ore market, yarn market.
(iii) Terminal market : The market where finished goods are bought and then sold to the ultimate consumers or the users of the product.
(5) On the basis of nature of goods:
(i) Commodity market : The market for goods, material or produce is called commodity market, e.g. market for consumer goods, industrial goods, etc.
(ii) Capital market : A market place where long term funds required by business enterprises are borrowed and lent is called capital market, The financial assets which are bought and sold in the capital market have long or indefinite maturity period. It deals in both debt and equity with maturity ranging from 1 year to 10 years.
(6) On the basis of regulation:
(i) Regulated market : Markets which are governed (regulated) and controlled by the statutory or legal provisions of the country are called regulated market, e.g. stock exchanges, foreign exchanges, commodity exchanges, etc.
(ii) Unregulated or free market : The markets which are usually regulated and operated according to the forces of demand and supply and not controlled by any statutory provisions are called unregulated or free market, e.g. market for various S commodities and services.
(7) On the basis of competition:
(i) Perfect market : A type of market in which large numbers of buyers and large numbers of sellers exist to buy and sell homogeneous product at prevailing market price is called perfect market. In this market, all buyers and all sellers have equal access to all information and have perfect knowledge about the market conditions. Neither single buyer nor a single seller can influence price. One uniform single price prevails in the market, This model of perfect market is frequently used in economic analysis.
(ii) Imperfect market : A type of market which has distinct features of market imperfection such as single seller, imperfect knowledge of market conditions on the part of buyers or sellers, failure to make adjustment in demand and supply, etc. is called imperfect market, e.g. monopoly market. Imperfect market is further sub-divided into the following categories:
(a) Monopoly : The word monopoly is the combination of two words, viz. ‘mono’ means one and ‘poly’ means seller. Accordingly, a market structure which is characterised by a single seller selling unique product in the market is called monopoly. In monopoly, seller faces no competition as he is the sole seller of goods with no close substitute. He controls the entire supply and enjoys the power of setting the price for his goods. He is price maker.
(b) Duopoly : A market situation in which two suppliers dominate the entire market for a commodity or service. In this market, there are two sellers, who either sell a homogeneous product or differentiated product. They enjoy a monopoly in the product produced and sold by them.
(c) Oligopoly : Oligopoly is a state of limited competition in which market is shared by a limited number (few) of producers or sellers producing and selling either a homogeneous product or a differentiated product.
(d) Monopsony : Market situation in which there is only one buyer substantially controls the market as major and single purchaser of goods and services offered by many producers or sellers, is called monopsony market, e.g. labour market, a firm is the sole purchaser of a certain kind of labour.
Question 3.
Explain in detail 7Ps of Marketing.
Answer:
7 Ps of marketing are explained as follows:
(1) Product : An article, goods, commodity, or service that is manufactured and offered to the customers for sale is called product. It has capacity to satisfy desire or need of the customers. The products may be either tangible or intangible. It can be in the form of goods or services. By undertaking extensive market research the business enterprises are required to decide the right type of products to be produced and sold. The product creates impact on the mind of the customers on which success or failure of business firm depends.
(2) Price : The amount of money given or required to buy a product is called price. It is essentially the amount that customer ready to pay for a product. The cost of the product, willingness of the customers to pay for the product, value and utility of the product are the main factors that businessmen have to considered while (determining) deciding the price of a product. Price of the product should be neither too high which affects the demand adversely nor too low which reduces the profitability of the business.
(3) Place : The distribution or placement of product is a significant aspect of the marketing, Place is the element of marketing mix that ensures that right product is distribute and made conveniently available to the potential consumers or buyers at right price, at right location and at right time too. The business enterprises are required to distribute the products at a place easily approachable to the potential consumers or buyers. Place in this respect covers location, distribution and the ways of giving the products in the hand of the customers. The system of distribution decides the coverage of the product in the market.
(4) Promotion : Promotion is one of the basic elements of marketing because it makes the customers aware of its product, creates brand recognition and sales. Promotion refers to any type of marketing communication used to inform and persuade potential buyers or consumers to buy the products by explaining them the regular merits of products, service, brand, etc. Promotion mix comprises of different tools such as advertising, sales promotion, direct marketing, personal selling, etc. promotional strategies to be used in the business depends on various factors such as budget, target market and the message wants to communicate.
(5) People : The employees, i.e. people inside the business and consumers/customers, i.e. people outside of the business create direct or indirect impact (influence) on the business. People include all individuals that play key role in offering the product or service to the buyer or customer. People inside the business comprise employees who assist to deliver services to the customers. Right people appointed to work at right place definitely add value to the business. The management or employer of the business enterprises are required to recruit right people, train them, develop their skill and retain them for their success.
(6) Process : The steps taken by the business organisation in carrying the products and services from the place of business to the doorsteps of customer are called process. Processes are significant to provide a quality service. Good process ensures same standard of service to the customers, save time and cost by enhancing efficiency. The growth and development of technology enable the business organisations in effective monitoring of the process of business and help to take corrective action if necessary.
(7) Physical environment : Marketing environment in which the interaction between customer and firm takes place is called physical environment. While providing (offering) intangible services, the service providers always try to incorporate certain tangible elements into their offering to increase experience of customers. In the service market, physical or tangible evidence is essential to ensure that the service is successfully provided. Physical evidence enables the customers to trace conveniently the brand leaders in the market. The physical evidence comprises of location, layout, packaging, branding, interior design, dress of the employees, their action, waiting area, etc.

Question 4.
Explain the functions of marketing in detail.
Answer:
The functions of marketing are explained as follows:
(1) Marketing research : The process of identifying the need and want of the customers, gathering information from the consumers, analysing and interpreting that information is called marketing research. Marketing research helps to assess the need in the market, requirements of consumers, time of purchases, quantity of purchases and prices at which products are bought and sold, etc. Marketing Research helps the business organisations to take various decisions on the marketing of products.
(2) Buying and assembling : Buying and assembling involve buying and bringing raw materials at one place for production purpose. This marketing functions is more important because quality and price of raw materials determine (fix) cost and quality of the final product.
(3) Market planning : After estimating the need of marketing the business enterprises are required to outiine the marketing plan and strategies to accomplish the predetermined aims and objectives. Marketing planning refers to the process of defining, determining and organising the marketing aims and objectives of the business and preparing strategies to accomplish those aims and objectives. In brief, market planning is nothing but a comprehensive blueprint that outlines overall marketing efforts of the business enterprises.
(4) Product development : In recent years, product development and design become an important function of marketing. It plays significant role in marketing (selling) the products. Every business organisation is required to develop its products to suit the needs of the consumers. Product design implies decisions in the areas of quality, colour, standards, shape, design, packing, etc. of the product. Most of the consumers always buy better and attractively designed products. Good and attractive design of the product also provides competitive benefits to the business by increasing its turnover. Product development is ongoing process because the needs of the consumers change as time passes.
(5) Standardisation and grading : Standardisation means setting up of standards or fixing certain norms in relation to design, quality, size, process, weight, colour, etc. of the product. Standardisation ensures uniformity in quality of products and helps to gain customers’ loyalty towards the product. Grading means physical sorting and classifying of the products according to standards set up, i.e. similar characteristics or features. Grading is completed on the basis of some features such as size, shape, quality, etc. Usually grading is done in the case of agricultural commodities like rice, wheat, potatoes, sugar, cotton, etc.
(6) Packing and labelling : Attractive package and label make the product successful and create long lasting impression on the consumers about the product. Designing the package for the product in attractive manner is called packaging, Packaging protects the product from breakage, leakage, damage, and destruction. Botties, plastic bags, wooden boxes, bubble bags, containers, tins, jute bags, packing foams, etc. are used for packing the products. A slip providing information of product and its producer pasted or affixed on the product container is called label. It gives protection to the product and serves as an effective tool of marketing.
(7) Branding : Branding means giving a name, symbol, mark or numeral to a product for the purpose of giving a distinct identity to that product is called branding. A brand which is registered is called Trademark. No one can copy trademark in marketing field. Branding gives the product a separate identity and recognition among the consumers which helps to expand business and increase brand awareness in the market.
(8) Customer support service : As the customer is regarded as centre point or the king of the market, business organisation must take required steps to satisfy the customers. The business organisations are also required to take every possible steps to render support services to the customers. Regular support services given to the customers increase their loyalty towards the business organisation. The support services like pre-sales services, after-sales services, consumer helpline, technical assistance, product demonstration, etc. are usually provided to the customers by the businessmen. Customer support services facilitate the business organisations to get, retain and increase the customers.
(9) Pricing of product : Pricing of product is an important and challenging functions of marketing and plays significant role in the market having cut-throat competition. Many times success or failure of products depend on the price charged for the product. While fixing the price of the product the business organisation is required to considered several factor such as cost, desired profit, price of the competitions product, market condition, demand for the product, etc. As per the requirement of market, the businessmen are required to change the prices of the products. The price fixed should neither be too high which may lose customers nor too low which may compel business to incur loss. Hence for deciding the right price, extensive market research should be undertaken.
(10) Promotional channels : The process of convening the consumers information of the products, their features, prices, uses, etc. and inducing them to buy the products is called promotion. Important tools of promotion include personal selling, advertisement, publicity and sales promotion. The businessmen can use some of the tools or combination of some tools for promotion of their products. Promotional activities increase brand awareness in the market.
(11) Distribution: The activities which are related to movement of finished products from the place of business to the doorsteps of consumers are called distribution. The distribution comprises of transportation, material handling, order processing, packaging, warehousing, inventory control, market forecasting, plant and warehousing location and customer service. The business organisation is required to spend major part of marketing budget of the business or the distribution. The significance of physical distribution for business organisation mostly depends on the type of product and level of customer satisfaction.
(12) Transportation : Physical movement of finished products as well as raw material from the s place of origin or production to the doorsteps of consumers is called transportation. Transportation is necessary because production, sale and consumption cannot take place at one place. By carrying the products from the place of plenty to place of scarcity the transport creates place utility into the products. The factors such as geographical boundaries, nature of products, cost, target ; market, speed, reliability, frequency, safety, etc. help to decide the mode of transport to be used. The modes of transport used by the business are categorised as road, air, water, railways, pipeline transport.
(13) Warehousing : Warehousing means storing of goods in a godown to held them in stock from the time of production or purchase till the time of their sale. Warehousing enables the business organisation to keep and maintain a smooth flow of goods by maintaining balance between supply and demand of the products warehousing helps to stabilizes the prices in the market. This marketing function is carried out by manufacturers, wholesalers and retailers. By holding the stock of products over certain period, warehousing creates time utility in the products.
Question 5.
Explain importance of marketing to the society and consumers.
Answer:
[A] Importance of marketing to the society:
(1) Increase in standard of living : The main aim of marketing is to supply goods and services to fulfil the customers’ requirements. Marketing finds out the requirements of the customers and accordingly make efforts to supply quality products at cheaper prices. This in turn helps to maintain and raise the standard of living of the customers. In recent era, large scale production of products and services have considerably reduced their prices due to which poor people also attain reasonable level of living.
(2) Provide employment : Modern marketing i undertakes almost all functions of organisation such as buying, selling, financing, transport, warehousing, risk bearing, research and development, etc. which generate and create more job opportunities in different capacities and help to solve the problem of unemployment.
(3) Decreases distribution coats : Effective and proper utilisation of channel of distribution reduces overall cost of the products and services, Thus, marketing activities make the goods and services available to the society at cheaper prices. Due to reduction in the cost, number of potential consumers will also increase. It also gives guarantee of timely availability of the product.
(4) Consumer awareness : Marketing helps the society by educating consumers and by giving information of new products and services available in the requirements of consumers by providing relevant information, goods and services to the people as per their demand and taste. Marketing also includes information to help in making a purchase.
(5) Increase in National income : The scientifically well organised marketing activities facilitates rapid economic development of the nation. Well organised and effective marketing of products and services facilitates industrialisation, increases job opportunities and develop the economy. Effective marketing integrates agriculture and industry which in turn bring rapid development in the country.
(6) Managing consumer expectations : Marketing research enables the business organisations to understand the requirements of the consumers which helps in development of products to satisfy the customers expectation. The business organisations make certain changes in the product by considering customers’ review. Through marketing, business enterprises inform consumers about the major changes such as mergers, transfer in ownership which affect product offerings. Government regulations stop marketers to make false and misleading claims.
[B] Importance of marketing to the consumers:
(1) Promotes product awareness : Many companies undertake different marketing activities to promote their products and services. Marketing makes consumers aware about the different products, various brands and features of products and services available in the market. Marketing facilitates the consumers to take right decision on purchases of products. On the basis of marketing information consumers can compare product features, availability, price and other essentials. Marketing also improves the quality of life of the consumers.
(2) Provides quality products : On account of increasing competition in the market, consumers easily get information about the product and services available in the market. Marketing creates moral pressure on the business enterprises to sell quality products and services to the consumers. If business enterprises supply defective products to the consumers, sooner or later, it will create negative image of the business and adversely affect customers’ loyalty towards business and products.
(3) Provides variety of products : Marketing provides information to the consumers about the product and attracts them to purchase the same market segment or marketing plan becoming critical in any business on account of increase in customer population their preferences and availability of competitive options. The business organisation are required to launch product by considering market segment. The business organisations are required to make available variety of goods to fulfil the requirements of different market segments of the consumers. According to price, size and quality of products, variety’ may change.
(4) Helps in selection : In the competitive markets, different variety of products with different brands are available. Marketing enables the consumers to select the best products and services from the different options available.

(5) Consumer satisfaction : The main and primary aim of marketing policy is to advertise and give assurance of good quality product to the consumer. When the expectations and requirements of customers are fulfilled, the customers become more comfortable and get satisfied. Marketing efforts result into customers’ satisfaction by way of honest advertising, assurance of quality products and accessibility of innovative products. In this manner, marketing makes efforts to give satisfaction to the consumers.
(6) Regular supply of goods : Regular supply of goods to the consumers is practicable through effective and efficient distribution channel of marketing. Marketing also helps to keep and maintain the balance between demand and supply. As a result, prices get stabilised.
OCM 12th Commerce Textbook Solutions Digest
![]()
![]()