Class 10th Sanskrit Aamod Chapter 9 धेनोाघः पलायते Question Answer Maharashtra Board
Balbharti Maharashtra State Board Class 10 Sanskrit Solutions Amod Chapter 9 धेनोाघः पलायते Notes, Textbook Exercise Important Questions and Answers.
Std 10 Sanskrit Chapter 9 Question Answer
Sanskrit Amod Std 10 Digest Chapter 9 धेनोाघः पलायते Textbook Questions and Answers
भाषाभ्यास:
1. पूर्णवाक्येन उत्तरं लिखत ।
प्रश्न अ.
चिमणराव: कस्यां मग्नः?
उत्तरम् :
चिमणराव: पत्रक्रीडायां मग्नः।

प्रश्न आ.
क्रीडायां को व्याघ्रं भल्लूकं च नाटयत:?
उत्तरम् :
क्रीडायां द्वौ बहुरूपधारिणौ नरौ व्यानं भल्लूकं च नाटयतः।
प्रश्न इ.
महिला केन मूर्च्छिता?
उत्तरम् :
महिला व्याघ्रस्य मनुष्यबाण्या भाषणेन मूर्छिता।
प्रश्न ई.
चिमणरावेण किं निश्चितम् ?
उत्तरम् :
सर्कसस्वामिन : साहाय्य करणीयम् इति चिमणरावेण निश्चितम्।
प्रश्न उ.
सर्कसक्रीडायाः प्रारम्भः कदा जात: ?
उत्तरम् :
सर्कसक्रीडाया: प्रारम्भः रात्रौ दशवादने जातः ।

प्रश्न ऊ.
चिमणराव: किमर्थं प्रयत्तवान् ?
उत्तरम् :
चिमणराव यावत् शक्यं जनान् रञ्जयितुं प्रयत्तवान्।
प्रश्न ए.
कैः आनन्देन तालिकावादनम् आरब्धम् ?
उत्तरम् :
उत्तेजितैः प्रेक्षकै: आनन्देन तालिकावादनम् आरब्धम्।
प्रश्न ऐ.
धेन्वा किं लक्षितम् ?
उत्तरम् :
धेनुना इमौ न परिचिती व्याघ्रभल्लूको इति लक्षितम्।
प्रश्न ओ.
भल्लूकः कुत्र आरूढवान् ?
उत्तरम् :
भल्लूक: पटमण्डपस्तम्भम् आरूढवान्।

प्रश्न औ.
प्रेक्षकाः कदा कोलाहलं कृतवन्त: ?
2. माध्यमभाषया उत्तरं लिखत ।
प्रश्न अ.
सर्कसस्वामी किमर्थं चिमणरावं प्रति आगतः ?
उत्तरम् :
‘धेनोर्व्याघ्रः पलायते।’ या कथेत चिमणरावांच्या स्वभाव वैशिष्ट्यांमुळे जी विनोदनिर्मिती होते त्यामुळे वाचकांच्या चेहऱ्यावर स्मितहास्य उमटते. त्याचप्रमाणे ही कथा म्हणजे एका प्रादेशिक भाषेतून प्राचीन भाषेतील अनुवादाचा उत्कृष्ट नमुना आहे.
या कथेचा नायक चिमणराव हा साधाभोळा, प्रामाणिक आणि देशभक्त आहे. त्यांनी ‘स्काऊट’ सारखाच शूरवीर मुलांचा संघ स्थापन केला, ज्याद्वारे लहान मुलांच्या मनात परोपकार, शौर्य, सहकार्य, समाजसेवेचे संस्कार रुजवण्याचा त्याचा मानस आहे.
सुट्टीमध्ये त्याने बनेश्वर नावाच्या गावात या मुलांच्या चमूला शिबिरासाठी नेले. शिबिराच्या पहिल्याच दिवशी दुपारचे जेवण आटपून चिमणराव मुलांसोबत पत्ते खेळण्यात मग्न असताना, नुकताच परिचय झालेले प्रोफेसर सर्कसवाले त्यांना शोधत आले. सर्कशीच्या मालकांना प्रयोगासाठी चिमणरावांचे सहकार्य अपेक्षित होते.
प्रयोगात अस्वल आणि वाघाचे सोंग घेणाऱ्या व्यक्ती भांडणादरम्यान जखमी झाल्या होत्या. त्यामुळे सर्कसच्या मालकांनी चिमणरावांना विनंती केली, की मुलांच्या संघातील दोघांना त्या दिवशीच्या प्रयोगात अस्वल व वाघाचे सोंग घेण्याची परवानगी द्यावी, जेणेकरुन प्रयोग न फसता यशस्वीपणे पूर्ण होईल.
The story ‘धेनोर्व्याघ्रः पलायते।’ brings smile on the faces of the readers due to the humor caused by innocent nature of चिमणराव. It is also an excellent example of translation skills from a regional language to the ancient language of India.
चिमणराव, the hero of this story isa sincere, innocent and patriotic person, made a troop of brave children like ‘scout’. The purpose behind it, was to inculcate values like benevolence, bravery, co-operation, social service etc. in the minds of the children.
During vacations, he took this troop of children to a village called ‘बनेश्वर for a camp. On the first day of the camp itself, when चिमणराव was busy playing cards with the children in the afternoon, प्रोफेसर सर्कसवाले, an owner of a circus came to meet him.
He was seeking help from चिमणराव in his circus show. The men enacting a bear and a tiger in his show were wounded. So the owner of the circus requested चिमणराव to allow any two soldiers from the troop to enact the animals and save the show from being a flop.

प्रश्न आ.
सर्कसक्रीडायाः आरम्भात् पूर्वं किं किम् अभवत् ?
उत्तरम् :
धेनोर्व्याघ्रः पलायते।’ या कथेत चिमणरावांच्या स्वभाव वैशिष्ट्यांमुळे जी विनोदनिर्मिती होते त्यामुळे वाचकांच्या चेहऱ्यावर स्मितहास्य उमटते. त्याचप्रमाणे ही कथा म्हणजे एका प्रादेशिक भाषेतून प्राचीन भाषेतील अनुवादाचा उत्कृष्ट नमुना आहे.
सर्कशीच्या मालकांनी प्रयोगासाठी सहकार्य करण्याच्या विनंतीचा चिमणरावांनी स्वीकार केला. त्यांनी आपल्या चमूतील बालवीरांपैकी अब्दुलची निवड अस्वलाच्या सोंगासाठी केली आणि ते स्वतः वाघाच्या वेशात तयार झाले. प्रयोग सुरु होण्यापूर्वी ते दोघे आपापले सोंग घेऊन नेमलेल्या पिंजऱ्यांत शिरले. वाघाचे सोंग घेतलेल्या चिमणरावांच्या पिंजऱ्यासमोर तोबा गर्दी जमली होती, त्यांनी प्रेक्षकांचे मनोरंजन करण्याचा प्रयत्न केला मात्र प्रेक्षक, ‘अरे, हा काय वाघ आहे? किती कृश आहे,’ अशी त्यांची खिल्ली उडवू लागले.
चिमणरावांनी वाघासारखी डरकाळी फोडल्यामुळे आईसोबत वाघ बघायला आलेला एक मुलगा घाबरला. तेव्हा त्याच्या आईन रागात वाघाला दगड फेकून मारला. परंतु त्यामुळे वाघाच्या वेशातील चिमणराव जखमी झाले. क्षणभर हे सगळे नाटक विसरून ते वेदनेने ओरडले, ‘पिंजऱ्यातल्या वाघाला बघून बायका दगड मारतात.’ त्यांचे हे वाक्य पूर्ण होण्याआधीच मनुष्यवाणीत बोलणाऱ्या वाघाला बघून ती बाई चक्रावली.
‘भूत आहे भूत’, असे किंचाळत ती चक्कर येऊन जमिनीवर पडली. त्यानंतर चिमणरावांचा मुलगा राषु पिंजऱ्याजवळ येऊन मधेच त्यांना म्हणाला, ‘आप्पा, तुम्हांला चहा प्यायचा आहे का?’ अशाप्रकारे वाघाला वडील म्हणून संबोधणारा मुलगा पाहून प्रेक्षक अधिकच संभ्रमात पडले. अशाप्रकारे, सर्कशीचा प्रयोग सुरु होण्यापूर्वीच तंबूत गडबड-गोंधळाचे वातावरण निर्माण झाले.
The story ‘धेनोक्नः पलायते।’ brings smile on the faces of the readers due to the humor caused by innocent nature of चिमणराव. It is also an excellent example of translation skills from a regional language to the ancient language of India. चिमणराव accepted the request of the circus owner to help him in his show.
He appointed अब्दुल, one of the cadets of his troop, as the bear and he himself got ready to enact the tiger in the show. Before the show would begin, both of them entered their allotted cages in the disguise of the bear and the tiger. A huge crowd. gathered around चिमणराव’s cage, who was disguised as the tiger.
He tried to entertain the crowd but the people mockingly said, “Is this a tiger? How weak is this!” A boy, who came to see the tiger along with his mother, got afraid due to the roaring of the tiger. So his mother threw a stone at him with anger. But behind the tiger’s disguise got wounded. For a second, he forgot about all the drama and screamed with pain, “Seeing the caged tigers, ladies throw stones.”
Before he could end his sentence, that lady got surprised seeing a tiger speaking in human voice. She fell on the ground being unconscious, screaming, “Ghost, it’s ghost!” Further चिमणराव’s son राघु came near the cage and asked him, “Father, would you like to have some tea?” With this the audience got even more confused. Thus, even before the circus show began, there was situation of chaos and confusion in the tent of circus.

प्रश्न इ.
पशूनां सहभोजनसमये क: हास्यपूर्णः अनवस्थाप्रसङ्गः समुत्पन्नः?
उत्तरम् :
‘धेनोव्याघ्रः पलायते।’ या कथेत चिमणरावांच्या स्वभाव वैशिष्ट्यांमुळे जी विनोदनिर्मिती होते त्यामुळे वाचकांच्या चेहऱ्यावर स्मितहास्य उमटते. त्याचप्रमाणे ही कथा म्हणजे एका प्रादेशिक भाषेतून प्राचीन भाषेतील अनुवादाचा उत्कृष्ट नमुना आहे. सर्कशीच्या मालकांच्या विनंतीचा स्वीकार करून चिमणराव व अब्दुलने अनुक्रमे वाघ व अस्वलाचे सोंग घेतले. प्रयोग रात्री 10 ला सुरू झाला. प्राण्यांच्या सहभोजनाचा लोकप्रिय कार्यक्रम 12 वाजता सुरू झाला.
प्रेक्षकांची उत्सुकता शिगेला पोहोचली. मंचावर तीन खुर्त्या आणि मधोमध एक गोलाकार टेबल ठेवण्यात आले. चिमणराव व अब्दुल आपापल्या खुर्चीत जाऊन बसले. त्या कार्यक्रमातील तिसरा प्राणी म्हणजे गाय सुद्धा मंचावर आली. चिमणराव आणि अब्दुल प्राण्यांचे सोंग घेऊन बसले होते, ती गाय मात्र खरीखुरी होते.
प्राण्यांसमोर खाद्य वाढले जात असता प्रेक्षकांनी आनंद आणि उत्सुकतेने टाळ्यांचा कडकडाट केला. त्या गायीला पूर्वी जे प्राण्यांचे सोंग घ्यायचे त्यांच्यासोबत कार्यक्रम करण्याचा सराव होता. परंतु थोड्याच वेळात गायीच्या लक्षात आले की, हे वाघ आणि अस्वल नेहमीचे नाहीत, ती संशय आणि गोंधळाने दोघांकडे पाहू लागली. आपली शिंगं उगारून ती दोघांवर गुरकावू लागली. चिमणरावांनी डरकाळी फोडून व आपले तीक्ष्ण दात दाखवून तिला घाबरवण्याचा प्रयत्न केला, परंतु ती त्याला बधली नाही. अब्दुलने हाताने तिला चापट मारली. तेव्हा चिडून ती दोघांवर धावून गेली.
प्रेक्षकांनी पाहिले की अस्वल घावरून तंबूच्या खांबावर चढले. हा त्यांना प्रयोगातील पूर्वनियोजित भाग आहे असे वाटले. परंतु अस्वल हातातून निसटल्यामुळे चिडून ती गाय आता चिमणराव म्हणजेच वाघावर धावून गेली. अशा संकटसमयी पूर्वी ठरवलेला सगळा बनाव आणि घेतलेले वाघाचे सोंग विसरून भितीने किंचाळत चिमणराव पळू लागले, ‘वाचवा, प्रोफेसरसाहेब, वाचवा!’ गायीला घाबरुन पळणारा वाघ पाहून प्रेक्षक गोंधळात पडले.पुढे त्यांना या नाटकाची कल्पना आली.
त्यांनी वाघाचे सोंग उतरवून चिमणरावांना बेदम मारले. इतके, की ते वेदनेने बेशुद्ध होऊन पडले. अशा प्रकारे चिमणराव प्रोफेसर सर्कसवाले यांना सर्कशीच्या प्रयोगासाठी सहकार्य करण्याचा प्रयत्न करत असताना प्रयोगाचा मात्र फज्जा उडाला.
The story ‘धेनोर्व्याघ्रः पलायते।’ brings smile on the faces of the readers due to the humor caused due to innocent nature of चिमणराव. It is also an excellent example of translation skills from a regional language to the ancient language of India.
Accepting the request of the circus owner, चिमणराव and अब्दुल were disguised as the tiger and the bear respectively. The circus began at 10 at night. The popular event of animal’s meal together began at 12. Audiences’ enthusiasm raised up. Three chairs and a circular stool in the middle were arranged on the stage. चिमणराव and अब्दुल took their seats. The cow, the third animal of the show, too came on the stage.
While चिमणराव and अब्दुल were disguised as the animals, the cow was a real one. As food was being arranged in front of the animals, the audience started clapping with joy and excitement. The cow was trained to perform in the show along with the earlier disguised men. But in a short while, she realised that these tiger and bear were not the usual ones.
She looked at both of them with confusion and doubt. Raising her horns she attacked them, चिमणराव tried to frighten her by roaring and displaying his sharp teeth, but she didn’t bother. अब्दुल beather with his hand. With this she got angry and ran over him. The audience saw that the bear climbed on the pole of the tent and they thought it was a part of the show. But the cow, being angry as the bear escaped, now attacked चिमणराव-the tiger.
Here चिमणराव forgot the planned drama and the tiger’s disguise due to fear and ran away screaming, “Help, professor, help!”. Seeing the tiger being afraid of a cow and running away, the audience got confused and then realised the drama. They removed the tiger’s disguise off चिमणराव’s body and beat him harshly, so much so that he fell unconscious with pain. Thus, चिमणराव tried to help प्रोफेसर सर्कसवाले in his show, but it turned out to be a fiasco.

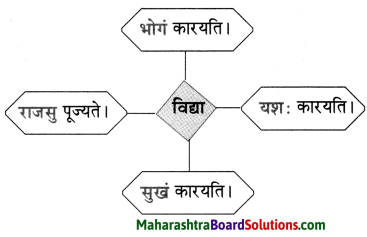
3. समानार्थकशब्दान् लिखत ।
मनः, गौः, व्याघ्रः, सैनिकः, क्रीडा, वेदना, भाषणम्, उत्कण्ठा, मनुष्यः, शृङ्गे, दन्ताः, उत्तमानम्
प्रश्न 1.
समानार्थकशब्दान् लिखत ।
मनः, गौः, व्याघ्रः, सैनिकः, क्रीडा, वेदना, भाषणम्, उत्कण्ठा, मनुष्यः, शृङ्गे, दन्ताः, उत्तमाङ्गम्
उत्तरम् :
- मग्नः – रतः, लीनः ।
- गौः – धेनुः, माहेयी, सौरभेयी, उस्रा, माता, शृङ्गिणी।
- व्याघ्रः – शार्दूल:, द्विपी ।
- सैनिकः – भटः, योध्दा, सेनारक्षः, योधः ।
- क्रीडा – खेलः।
- वेदना – संवेदः, निर्वेशः, उपलम्भः।
- उत्कण्ठा – उत्सुकता।
- भाषणम् – व्याहारः, भाषितम्।
- मनुष्यः – नरः, मानुषः, मर्त्यः, मनुजः, मानवः ।
- शृङ्गे – ककुदे, विषाणे।
- दन्ताः – रदनाः, दशना:।
- उत्तमाङ्गम् – मस्तकम्, शिरः, शीर्षम्, मूर्धा।

4. विरुद्धार्थकशब्दान् लिखत ।
समाप्य, प्राक्, विस्मृत्य, कृशः
प्रश्न 1.
विरुद्धार्थकशब्दान् लिखत ।
समाप्य, प्राक्, विस्मृत्य, कृशः
उत्तरम् :
- समाप्य × आरभ्य ।
- प्राक् × अनन्तरम्।
- विस्मृत्य × स्मृत्वा ।
- कृशः × बलवान, बलिष्ठः।
5. प्रश्ननिर्माणं कुरुत।।
प्रश्न अ.
अहं बालैः सह पत्रक्रीडायां मनः आसम् ।
उत्तरम् :
अहं कै: सह पत्रक्रीडायां मग्न: आसम्?

प्रश्न आ.
धेनुः वास्तविकी एव ।
उत्तरम् :
माहेयी / सौरभेयी / गौः / उस्रा / माता / शृङ्गिणी तु वास्तविकी एव।
प्रश्न इ.
सर्कसस्वामिनः विवशता मया अवगता ।
उत्तरम् :
कस्य विवशता मया अवगता?
प्रश्न ई.
भल्लूकवेशे अब्दुलः शोभेत ।
उत्तरम् :
भल्लूकवेषे कः शोभेत?
प्रश्न उ.
महिला मयि पाषाणखण्डान् अक्षिपत् ।
उत्तरम् :
महिला मयि कान् अक्षिपत्?

प्रश्न ऊ.
गौः मञ्चं समागता ।
उत्तरम् :
का मञ्चं समागता?
6. क: कं वदति ?
प्रश्न अ.
‘विज्ञापनं कृत्वा प्रवेशपत्राणि अपि विक्रीतानि ।’
उत्तरम् :
सर्कसक्रीडास्वामी लेखकं चिमणरावं वदति।
प्रश्न आ.
किं धेनुरपि …?’
उत्तरम् :
लेखक : चिमणराव : सर्कसक्रीडास्वामिनं वदति।
प्रश्न इ.
‘चायं पातुम् इच्छति किम्?’
उत्तरम् :
राघु: चिमणरावं/ लेखकं वदति।

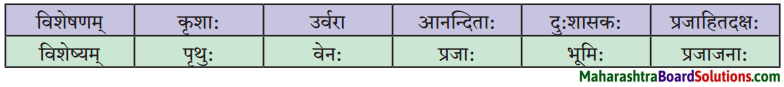
7. विशेषण-विशेष्याणां मेलनं कुरुत ।
प्रश्न 1.
विशेषण-विशेष्याणां मेलनं कुरुत ।
| विशेषणम् |
विशेष्यम् |
| लोकप्रियः |
आकर्षणम् |
| सर्वोच्चम् |
शुल्कम् |
| बहुरूपधारिणी |
व्याघ्रान् |
| अधिकम् |
प्रेक्षकाः |
| अधिक: |
प्रेक्षकेषु |
| भीत: |
सहभोजनकार्यक्रमः |
| पञ्जरस्थितान् |
नरौ |
| सम्भ्रान्ताः |
सम्पर्दः |
| त्रीणि |
व्याघ्रभल्लूको |
| निमग्रेषु |
बाल: |
| परिचितौ |
आसनानि |
उत्तरम् :
| विशेषणम् |
विशेष्यम् |
| लोकप्रियः |
सहभोजनकार्यक्रमः |
| सर्वोच्चम् |
आकर्षणम् |
| बहुरूपधारिणी |
नरौ |
| अधिकम् |
शुल्कम् |
| अधिक: |
सम्पर्दः |
| भीत: |
बाल: |
| पञ्जरस्थितान् |
व्याघ्रान् |
| सम्भ्रान्ताः |
प्रेक्षकाः |
| त्रीणि |
आसनानि |
| निमग्रेषु |
प्रेक्षकेषु |
| परिचितौ |
व्याघ्रभल्लूको |
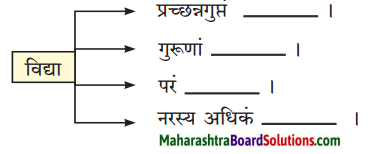
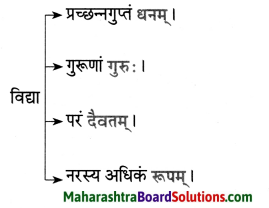
8. अमरकोषात् शब्दं प्रयुज्य वाक्यं पुनर्लिखत ।
प्रश्न अ.
धेनुः तु वास्तविकी एव ।
उत्तरम् :
धेनुः कीदृशी?

प्रश्न आ.
मम उत्तमाङ्गम् उपचारपट्टिकाभिः विभूषितम् ।
उत्तरम् :
मममस्तकम् / शिरः/ शीर्षम् / मूर्धा / मस्तक: उपचारपट्टिकाभिः विभूषितम्।
9. सूचनानुसारं कृती: कुरुत ।
प्रश्न अ.
पत्रक्रीडायां मनः आसम् अहम् । (बहुवचने लिखत।)
उत्तरम् :
पत्रक्रीडायां मग्नाः आस्म वयम्।
प्रश्न आ.
जनाः न सन्तुष्टाः । (एकवचने लिखत)
उत्तरम् :
जन: न सन्तुष्टः।

प्रश्न इ.
प्रेक्षकाः मां निघृणं ताडितवन्तः । (वाच्यपरिवर्तनं कुरुत ।)
उत्तरम् :
प्रेक्षकैः अहं निघृणं ताडितः।
Sanskrit Amod Class 10 Textbook Solutions Chapter 9 धेनोाघः पलायते Additional Important Questions and Answers
अवबोधनम्
(क) उचितं कारण चित्वा वाक्यं पुनर्लिखत।
प्रश्न 1.
सर्कस-क्रीडारङ्गस्य स्वामी लेखकम् अन्विष्यन् शिबिरं समागत: यतः ………….
(अ) सः लेखकस्य साहय्यम् इच्छति।
(ब) सः लेखक सर्कसक्रीडां द्रष्टुं निमन्वितवान्।
उत्तरम् :
(अ) लेखकस्य साहाय्यम् इच्छति।
प्रश्न 2.
सर्कसक्रीडायां व्याघ्रभल्लूको द्विपादौ एव यतः ……………
(अ) वास्तविको व्याघ्रभल्लूको पालयितुं तेषां सामर्थ्य नासीत्।
(ब) स: वास्तविक-व्याघ्रभल्लूकाभ्यां भीतः।
उत्तरम् :
(अ) वास्तविको व्याघ्रभल्लूको पालयितु तेषां सामथ्र्य नासीत्।
प्रश्न 3.
अब्दुल: भल्लूकपात्रे नियोजित: यतः ……….
(अ) लेखक: भल्लूकपात्रम् अभिनेतुं नैच्छत्।
(ब) अब्दुल: भल्लूकवेषे शोभेत इति लेखक: अमन्यत।
उत्तरम् :
(अ) अब्दुल: भल्लूकवेधे शोभेत इति लेखक: अमन्यत ।

प्रश्न 4.
माता व्याघ्र पाषाणखण्डान् क्षिपति यतः
(अ) तस्याः पुत्रः व्याघ्रगर्जनां श्रुत्वा भीतः।
(ब) माता व्याघ्र पीडयितुम् इच्छति स्म।
उत्तरम् :
(अ) तस्याः पुत्र : व्याघ्रगर्जनां श्रुत्वा भीतः।
प्रश्न 5.
माता मूच्छिता यतः ………
(अ) माता ज्वरपीडिता।
(ब) तस्याः पुरत: व्याघ्रः मनुष्यवाण्या अवदत्।
उत्तरम् :
(ब) तस्याः पुरत: व्याघ्रः मनुष्यवाण्या अवदत्।
प्रश्न 6.
कार्यक्रमस्य अन्ते प्रेक्षका: व्याघ्रवेषधारिणं मनुष्यं ताडितवन्तः यतः
(अ) व्याघ्रवेषधारिणा मनुष्येण चौर्यकार्य कृतम्।
(ब) तेन मनुष्येण व्याघ्रवेषं धृत्वा प्रेक्षकाणां वञ्चना कृता।
उत्तरम् :
(ब) तेन मनुष्येण व्याघ्रवेषं धृत्वा प्रेक्षकाणां वनना कता।

(ख) उचितं पर्यायं चित्वा वाक्यं पुनर्लिखत।
प्रश्न 1.
1. तथा विज्ञापनं कृत्वा …………….. विक्रीतानि। (प्रवेशपत्राणि/पिहितपत्राणि)
2. अस्माकं सर्कसक्रीडायां …………….., भल्लूक: च द्विपादौ एव। (गौः/ व्याघ्रः)
उत्तरम् :
1. प्रवेशपत्राणि
2. व्याघः
प्रश्न 2.
- ……….. भल्लूकवेषे नियोजितः । (लेखक:/अब्दुल:)
- व्याघ्रस्य अभिनयाथै ……. सिद्धः । (लेखक:/अब्दुल:)
- ……….. पारस्य पुरत: सम्मदः जातः । (व्याघ्रस्य/ भल्लूकस्य)
- महिला पुत्रेण सह ……….. द्रष्टुम् आगता। (व्याघ्र/भल्लूकं)
उत्तरम् :
- अब्दुल:
- लेखक
- व्याघ्रस्य
- व्याधं

प्रश्न 3.
1. ……… मनुष्यवाण्या भाषणेन सा महिला मूर्छिता। (भल्लूकस्य / व्यावस्य)
2. ……. क्रीडाया: चरमबिन्दुः समायातः। (द्वादशवादने / दशवादने)
उत्तरम् :
1. व्याघ्रस्य
2. द्वादशवादने
प्रश्न 4.
- सम्भ्रमेण संशयेन च ……… दृष्टवती। (आवां / वयम्)
- अब्दुल: तां ………… ताडितवान्। (हस्तेन / हस्तात)
- प्रेक्षका: मम व्याघ्रवेषं निष्कास्य मां ………. ताडितवन्तः। (निपुणं / सस्नेह)
उत्तरम् :
- आवां
- हस्तेन
- निघणं
(ग) कः कं वदति।
प्रश्न 1.
किं प्राणिनो: कलहः?
उत्तरम् :
लेखक: चिमणराव: सर्कसक्रीडास्वामिनं वदति।

प्रश्न 2.
नहि, नहि, धेनुस्तु वास्तविकी एव।
उत्तरम् :
सर्कसक्रीडास्वामी लेखक चिमणरावं वदति।
प्रश्न 3.
कृपया साहाय्यं करोतु।
उत्तरम् :
सर्कसक्रीडास्वामी लेखकं चिमणरावं वदति।
(घ) पूर्णवाक्येन उत्तरं लिखत।
प्रश्न 1.
एषः गद्यांश: कस्मात् पाठात् उद्धृतः?
उत्तरम् :
एषः गद्यांश: ‘धेनोर्व्याघ्र : पलायते’ इति पाठात् उद्धृतः ।

प्रश्न 2.
भल्लूकपात्रे क: नियोजितः?
उत्तरम् :
भल्लूकपात्रे अब्दुल : नियोजितः।
प्रश्न 3.
कुत्र सम्मः जातः?
उत्तरम् :
व्याघ्रपञ्जरस्य पुरत: सम्मर्द जातः ।
प्रश्न 4.
का पाषाणखण्डान् अक्षिपत्?
उत्तरम् :
व्याघ्र द्रष्टुं स्वपुत्रेण सह आगता माता पाषाणखण्डान् अक्षिपत्।

प्रश्न 5.
कः धेनुं हस्तेन ताडितवान्?
उत्तरम् :
अब्दुल: धेनुं हस्तेन ताडितवान्।
प्रश्न 6.
कदा प्रेक्षागारे मझन् कोलाहल: उत्पन्नः?
उत्तरम् :
धेनो: भीतं, पलायमानं व्याघ्रं दृष्ट्वा पेक्षागारे महान् कोलाहल: उत्पनः ।
(च) वाक्यं पुनलिखित्वा सत्यम् / असत्यम् इति लिखत।
प्रश्न 1.
1. सर्कसक्रीडायां लोकप्रियः सङ्गीतकार्यक्रमः प्रचलति।
2. बहुरूपधारिणौ नरौ गोव्याघ्रौ नाटयतः।
उत्तरम् :
1. असत्यम्।
2. असत्यम्।

प्रश्न 2.
- व्यानवेषे अब्दुलः समुपस्थितः।
- व्याघ्रवेषे लेखक: समुपस्थितः।
- महिला भल्लूकं द्रष्टुम् आगता।
- व्याघ्रस्य गर्जनया बाल: आनन्दितः।
उत्तरम् :
- असत्यम्।
- सत्यम्।
- असत्यम्।
- असत्यम्।
प्रश्न 3.
- रात्रौ दशवादने सर्कसक्रीडायाः प्रारम्भः जातः ।
- श्रोतृवृन्दः लेखने मग्नः।
- पूर्व व्याघ्रभल्लूको वास्तविकौ आस्ताम्।
उत्तरम् :
- सत्यम्।
- असत्यम्।
- असत्यम्।

प्रश्न 4.
1. धेनु: व्याघ्रात् भीता।
2. प्रेक्षका; भल्लूकवेषधारिणम् अब्दुलं ताडितवन्तः।
उत्तरम् :
1. असत्यम्।
2. असत्यम्।
शब्दज्ञानम् ।
(क) सन्धिविग्रहः।
- धेनोर्व्याघः – धेनोः + व्याघ्रः।
- मामेव – माम् + एव।
- व्याघ्रश्च – व्याघ्रः + च।
- इत्येतेषाम् – इति + एतेषाम्।
- द्वावपि . द्वौ + अपि।
- नास्ति – न + अस्ति ।
- धेनुरपि – धेनु: + अपि।
- धेनुस्तु – धेनुः + तु।
- करणीयमिति – करणीयम् + इति।
- प्रागेव – प्राक् + एव।
- यावच्छक्यम् – यावत् + शक्यम्।
- किमयम् – किम् + अयम्।
- कृशोऽयम् – कृशः + अयम्।
- पूर्वमेव – पूर्वम् + एव।
- भूतोऽयम् – भूतः + अयम्।
- पूर्वमपि – पूर्वम् + अपि।
- तथापि – तथा + अपि ।
- अचिरादेव – अचिरात् + एव।
- अहमपि – अहम् + अपि।
- किञ्चिदपि – किञ्चित् + अपि ।
- मूलस्वरूपमाश्रितवान् – मूलस्वरूपम् + आश्रितवान्।

(ख) त्वान्त-ल्यबन्त-तुमन्त-अव्ययानि ।
1.
| त्वान्त अव्यय धातु + त्वा / ध्या / ट्वा / ड्वा / इत्वा / अयित्वा |
ल्यबन्त अव्यय उपसर्ग + धातु + य / त्य |
तुमन्न अव्यय धातु + तुम् / धुम् / टुम् / दम् / इतुम् / अयितुम् |
| कृत्वा, धृत्वा |
समाप्य |
पालयितुम् |
| यितुम् मत्वा, धृत्वा, दृष्ट्वा |
विस्मृत्य |
रञ्जयितुम्, द्रष्टुम् |
2.
| ल्यबन्त अव्यय उपसर्ग + धातु + य / त्य |
तुमन्त अव्यय धातु + तुम् / धुम् / टुम् / दुम् / इतुम् / अयितुम् |
| आगत्य |
पातुम, कर्तुम् |

(ख) त्वान्त – ल्यबन्त – अव्ययानि।
| तुमन्त अव्यय धातु + त्वा / ध्वा / ट्वा / दवा / इत्वा / अयित्वा |
ल्यबन्त अव्यय उपसर्ग + धातु + य / त्य |
| कृत्वा, दृष्ट्वा |
उन्नम्य, विचिन्त्य, विस्मृत्य, निष्कास्य |
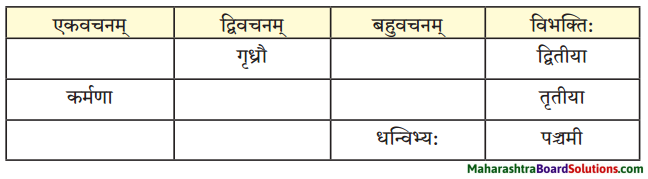
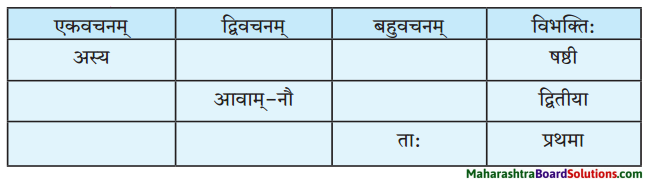
(ग) विभक्त्यन्तरूपाणि।
द्वितीया – भोजनादिकम्, माम, विज्ञापनम, व्याघ्रम्, भल्लूकम्, साहाय्यम्, व्याघ्रभल्लूकवेषौ, जनान, व्याघम्, पाषाणखण्डान्, पञ्जरस्थितान्, व्याघान्, त्रीणि, आसनानि, वर्तुलाकारम्, पीठम्, मञ्चम्, आवाम्, शृङ्गे, नौ, तीक्ष्णदन्तान, ताम, तम्, पटमण्डपस्तम्भम, व्याघ्रम, माम, लक्ष्यम्, व्याघ्रत्वम्, द्विपादम, मूलस्वरूपम्, भीतम्, पलायमानम्, पुण्यपत्तनदिशम्।
तृतीया – बालैः, प्राध्यापकमहाशयेन, मया, मया, यदृच्छया, उपहासेन, स्वपुत्रेण, गर्जनया, वेदनया, मनुष्यवाण्या, भाषणेन, उच्चैः, उत्तेजितैः, प्रेक्षकैः, आनन्देन, ताभ्याम्, धेनुना, सम्भमेण, संशयेन, हस्तेन, तेन, चापल्येन, भीत्या, वेदनाभिः, उपचारपट्टिकाभिः।
पञ्चमी – कतिपयेभ्यः, मासेभ्यः, कलहकारणात्, अचिरात्, तस्याः, धेनोः।
षष्ठी – शिबिरस्य, अस्माकम्, एतेषाम, पशूनाम्, प्रयोगस्य, भल्लूकयोः, प्राणिनोः, भवतः, प्रेक्षकाणाम्, सर्कसस्वामिनः, तस्य, परोपकारस्य, व्याघ्रस्य, मम, पारस्य, मम, वाक्यसमाप्तेः, व्याघ्रस्य, सर्कसक्रीडाया:, प्रेक्षकाणाम्, उत्कण्ठायाः, अस्माकम्, खाद्यस्य, सहभोजनस्य, भल्लूकवेषे, भल्लूकपात्रे, स्वपञ्जरयोः, मयि, भूमौ, रात्रौ, दशवादने, द्वादशवादने, मझे, मध्यभागे, एकस्मिन्, आसने, प्रारम्भे, जाते।
सप्तमी – प्रथमदिने, मध्याहे, पत्रक्रीडायाम, सर्कसक्रीडायाम्।
सम्बोधनम् – कॅप्टनसाहेब, महाशय।

(घ) विशेषण – विशेष्य – सम्बन्धः।
| विशेषणम् |
विशेष्यम् |
| 1. कतिपयेभ्यः |
मासेभ्यः |
| 2. द्विपादौ |
भल्लूको |
| 3. वास्तविकी |
धेनुः |
| 4. महान् |
उपकार: |
| 5. मनुष्यवाण्या |
भाषणेन |
| 6. मूर्छिता |
महिला |
| 7. वर्तुलाकारम् |
पीठम् |
| 8. भल्लूकवेषधारी |
अब्दुल: |
| 9. उत्तेजितैः |
प्रेक्षकैः |
| 10. अभ्यस्ता |
धेनुः |
| 11. सन्तुष्टाः |
जनाः |
| 12. कृशः |
व्याघ्रः |
| 13. पूर्वनियोजिता |
क्रीडा |
| 14. प्रमुदिताः |
प्रेक्षका: |
| 15. क्रुद्धा |
धेनुः |
| 16. चतुष्पादविशिष्टम् |
व्याघ्रत्वम् |
| 17. द्विपादम् |
मूलस्वरूपम् |
| 18. भीतम्, पलायमानम् |
व्याघ्रम् |
| 19. महान् |
कोलाहल: |
| 20. अनुत्तमानि |
अङ्गानि |

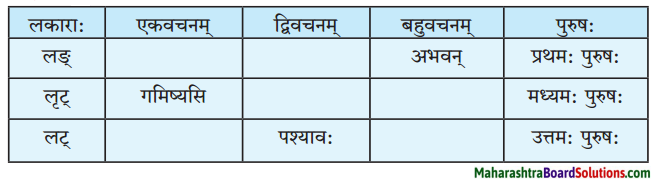
(च) लकारं लिखत।
प्रश्न 1.
- पत्रक्रीडायां मग्न: आसम् अहम्।
- शृणोतु कॅप्टनसाहेब।
- बहुरूपधारिणौ नरौ व्याघ्रभल्लूको नाटयतः।
- अहं प्रार्थये।
- महान् उपकार: स्यात्।
- कृपया साहाय्यं करोतु।
उत्तरम् :
- लङ्लकार :
- लोट्लकार :
- लट्लकार:
- लट्लकार:
- विधिलिङ्लकार:
- लोट्लकार:

प्रश्न 2.
- भल्लूकवेषे अब्दुल: शोभेत।
- जनाः उपहासेन अवदन्।
- महिला: क्षिपन्ति पाषाणखण्डान्।
उत्तरम् :
- विधिलिङ्लकार:
- लङ्लकार:
- लट्लकार:
प्रश्न 3.
1. सा शृङ्गे उन्नम्य नौ आक्रामत्।
2. प्राध्यापकमहाशय, परित्रायताम्।
उत्तरम् :
1. लङ्लकारः
2. लोट्लकार:
(छ) प्रश्ननिर्माणं कुरुत।
प्रश्न 1.
प्राध्यापकमहाशयेन रहस्यभेद: कृतः।
उत्तरम् :
केन रहस्यभेदः कृतः? ।

प्रश्न 2.
बहुरूपधारिणौ नरौ व्याघ्रभल्लूको नाटयतः।
उत्तरम् :
को व्याघ्रभल्लूको नाटयतः?
प्रश्न 3.
व्याघ्रस्य मनुष्यवाण्या भाषणेन सा महिला मूर्छिता।
उत्तरम् :
केन कारणेन सा महिला मूर्छिता?
प्रश्न 4.
रात्रौ दशवादने सर्कसक्रीडायाः प्रारम्भः जातः ।
उत्तरम् :
कस्मिन् समये / कदा सर्कसक्रीडाया: प्रारम्भः जातः?
प्रश्न 5.
क्रुद्धा धेनु: व्याघ्र लक्ष्यं कृतवती।
उत्तरम् :
क्रुद्धा धेनुः कं लक्ष्यं कृतवती?

प्रश्न 6.
प्रेक्षागारे महान् कोलाहल: उत्पन्नः ।
उत्तरम् :
कुत्र महान् कोलाहल: उत्पन्नः?
पृथक्करणम्
क्रमेण योजयत।
प्रश्न 1.
- शिबिरस्य प्रथमं दिनम्।
- सर्कसस्वामिनः साहाय्ययाचना।
- सहभोजनस्य रहस्यभेदः।
- सर्कसस्वामिनः आगमनम्।
उत्तरम् :
- शिबिरस्य प्रथमं दिनम्।
- सर्कसस्वामिनः आगमनम्।
- सहभोजनस्य रहस्यभेदः।
- सर्कसस्वामिनः साहाय्ययाचना।

प्रश्न 2.
- माता व्याघ्र पाषाणखण्डान् अक्षिपत्।
- व्याघ्रस्य पुरत: सम्मर्दः जातः।
- व्याघ्रस्य मनुष्यवाण्या भाषणेन महिला मूर्छिता।
- व्याघ्रस्य गर्जनया बाल: भीतः।
उत्तरम् :
- व्याघ्रस्य पुरतः सम्मदः जातः।
- व्याघ्रस्य गर्जनया बाल: भीतः।
- माता व्याघ्र पाषाणखण्डान् अक्षिपत्।
- व्याघ्रस्य मनुष्यवाण्या भाषणेन महिला मूर्च्छिता।
प्रश्न 3.
- भल्लूकवेषधारिण: अब्दुलस्य आगमनम्।
- सर्कसक्रीडाया: प्रारम्भः ।
- उत्तेजितैः प्रेक्षकैः तालिकावादनम्।
- पशूनां खाद्यस्य योजना।
उत्तरम् :
- सर्कसक्रीडायाः प्रारम्भः ।
- भल्लूकवेषधारिणः अब्दुलस्य आगमनम्।
- पशूनां खाद्यस्य योजना।
- उत्तेजितै: प्रेक्षकै; तालिकावादनम्।

प्रश्न 4.
- भल्लूकस्य पटमण्डपस्तम्भम् आरोहणम्।
- धेनोः सम्भ्रमः आक्रमणं च।
- चिमणरावस्य व्याघ्रत्वं विस्मृत्य द्विपादरूपाश्रयः ।
- सहभोजनस्य प्रारम्भः।
उत्तरम् :
- सहभोजनस्य प्रारम्भः।
- धेनोः सम्भ्रम: आक्रमणं च।
- भल्लूकस्य पटमण्डपस्तम्भम् आरोहणम्।
- चिमणरावस्य व्याघ्रत्वं विस्मृत्य द्विपादरूपाश्रयः ।

भाषाभ्यास:
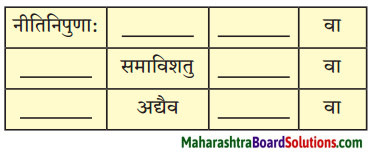
योग्य रूपं लिखित्वा रिक्तस्थानपूर्ति कुरुत।
प्रश्न 1.
- क्रीडायां ……….. नरौव्याघ्रभल्लूकौनाटयतः (द्वि-सङ्ख्यावाचक)
- ………… सैनिकौ प्रेक्षकाणां मनोरञ्जनं कुर्याताम्। (द्वि-सजावाचक)
- ………… महिला स्वपुत्रेण सह व्यानं द्रष्टुम् आगता। (एक – सङ्ख्यावाचक)
- राघुः पञ्जरसमीपम् आगत्य पृष्टवान्। (एक-आवृत्तिवाचक)
- सेवक: मझे “आसनानि स्थापितवान्। (त्रि-सङ्ख्यावाचक)
- अहम् “आसने उपवेशितः। (एक – सङ्ख्यावाचक)
उत्तरम् :
- द्वौ
- द्वौ
- एका
- एकवारं
- त्रीणि
- एकस्मिन्

सूचनानुसारं कृती: कुरुत।
(क) वाच्यपरिवर्तनं कुरुत।
प्रश्न 1.
अहं विरोधं दर्शितवान्।
उत्तरम् :
मया विरोध: दर्शितः।
प्रश्न 2.
सेवक: त्रीणि आसनानि स्थापितवान्।
उत्तरम् :
सेवकेन त्रीणि आसनानि स्थापितानि।
प्रश्न 3.
अब्दुल: तां हस्तेन ताडितवान्।
उत्तरम् :
अब्दुलेन सा हस्तेन ताडिता।

(ख) त्वान्त / ल्यबन्त अव्ययं निष्कासयत।
प्रश्न 1.
अहं व्याघ्रः न वदति इति विस्मृत्य अवदम्।
उत्तरम् :
अहं व्याघ्रः न वदति इति व्यस्मरम् अवदम् च ।
प्रश्न 2.
अहं गर्जनां कृत्वा तीक्ष्णदन्तान् दर्शितवान्।
उत्तरम् :
अहं गर्जनां कृतवान् तीक्ष्णदन्तान् दर्शितवान् च ।
(ग) स्म निष्कासयत।
प्रश्न 1.
जनाः वन्यपशून् द्रष्टुम् आगच्छन्ति स्म।
उत्तरम् :
जना: वन्यपशून् द्रष्टुम् आगच्छन् ।

(घ) भवान् स्थाने त्वं योजयत।
प्रश्न 1.
भवान् सहाय्यं करोतु ।
उत्तरम् :
त्वं साहाय्यं कुरु।
प्रश्न 2.
भवान् चायं पातुम् इच्छति किम्?
उत्तरम् :
त्वं चायं पातुम् इच्छसि किम्?
प्रश्न 3.
भवान् परित्रायताम्।
उत्तरम् :
त्वं परित्राबस्व।

(च) वचनं परिवर्तयत।
प्रश्न 1.
पत्रक्रीडायां मग्नः आसम् अहम्। (बहुवचने परिवर्तयत।)
उत्तरम् :
पत्रक्रीडायां मग्नाः आस्म वयम्।
प्रश्न 3.
एका महिला आगता। (द्विवचने परिवर्तयत।)
उत्तरम् :
द्वे महिले आगते।
(छ) लकारं परिवर्तयत।
प्रश्न 1.
पत्रक्रीडायां मग्नः आसम् अहम्। (विधिलिङ्लकारे परिवर्तयत।)
उत्तरम् :
पत्रक्रीडायां मग्नः स्याम् अहम्।

प्रश्न 2.
महान् उपकारः स्यात्। (लङ्लकारे परिवर्तयत।)
उत्तरम् :
महान् उपकारः आसीत्।
प्रश्न 3.
भल्लूकवेषे अब्दुल: शोभेत। (लङ्लकारे परिवर्तयत।)
उत्तरम् :
भल्लूकवेषे अब्दुलः अशोभत।
प्रश्न 4.
धेनुः नौ आक्रामत्। (विधिलिङ्लकारे परिवर्तयत।)
उत्तरम् :
धेनु: नौ आक्रामेत्।
(ज) वाक्यं शुद्धं कुरुत।
प्रश्न 1.
द्वौ नरौ व्याघ्रभल्लूको नाटयन्ति।
उत्तरम् :
द्वौ नरौ व्याघ्रभल्लूको नाटयतः।

प्रश्न 2.
मम पञ्जरात् पुरत: सम्मदः जातः ।
उत्तरम् :
मम पञ्जरस्य पुरतः सम्मर्दः जातः ।
प्रश्न 3.
धेनुः तयोः सह कार्यक्रमः कर्तुम् अभ्यस्ता आसीत्।
उत्तरम् :
धेनुः ताभ्यां सह कार्यक्रमः कर्तुम् अभ्यस्ता आसीत्।
प्रश्न 4.
कृद्धाः धेनु: मां लक्ष्यं कृतवती।
उत्तरम् :
कद्धा धेनु: मां लक्ष्यं कृतवती।

समासाः
| समस्तपदम् |
अर्थ: |
समासविग्रहः |
समासनाम |
| व्याघ्रभल्लूको |
tiger and bear |
व्याघ्रः च भल्लूक: च। |
इतरेतर द्वन्द्व समास |
| चरमबिन्दुः |
peak point |
चरम: बिन्दुः। |
कर्मधारय समास |
| तीक्ष्णदन्ताः |
sharp teeth |
तीक्ष्णा: दन्ताः। |
कर्मधारय समास |
| उत्तमाङ्गम् |
topmost organ |
उत्तमम् अङ्गम्। |
कर्मधारय समास |
| अभिनयार्थम् |
for enacting |
अभिनयाय इदम्। |
चतुर्थी तत्पुरुष समास |
| बालवीरचमः |
a troop of brave children |
बालवीराणां चमूः। |
षष्ठी तत्पुरुष समास |
| पाषाणखण्डा: |
pieces of stone |
पाषाणस्य खण्डाः। |
षष्ठी तत्पुरुष समास |
| लोकप्रियः |
dear amongst people |
लोकेषु प्रियः। |
सप्तमी तत्पुरुष समास |
| भल्लूकवेषधारी |
a man disguised as a bear |
भल्लूकवेषं धारयति इति। |
उपपद तत्पुरुष समास |
| अनुत्तमानि |
not excellent |
न उत्तमानि। |
नञ्तत्पुरुष समास |
धेनोाघः पलायते Summary in Marathi and English
प्रस्तावना :
‘धेनोव्याघ्रः पलायते।’ हे शीर्षक संस्कृत साहित्यातील भोजराजाच्या कथेतील एका प्रसिद्ध समस्येवर आधारीत आहे. ती म्हणजे ‘मृगात् सिंहः पलायते।’ या दोन्ही वाक्यांमध्ये लेखकांनी सृष्टीनियमांविरुद्ध अशा परिस्थितीचे चित्रण केले आहे. परंतु त्यातूनच निर्माण होणारा विनोद वाचकांच्या चेहऱ्यावर स्मितहास्य आणतो.
ही कथा मराठी साहित्यातील अजरामर कलाकृती असलेल्या ‘चिमणरावांचे चव्हाट’ या कथा संग्रहातील ‘कॅप्टन चिमणराव – स्काउटमास्तर’ या कथेचा अनुवाद असून संस्कृत वार्षिक अंक ‘समर्पणम्’ मधून प्रसिद्ध झाली होती. या कथेचा नायक व मराठी साहित्यातील प्रसिद्ध पात्र असलेला चिमणराव स्काऊटच्या धर्तीवर बालवीरांचा गट निर्माण करु इच्छितो.
परोपकार, शूरता, समाजसेवा इ. मूल्यांचे महत्त्व त्या लहान मुलांच्या मनावर बिंबवणे हा त्यामागचा शुद्ध हेतू. या बालचमूला सुट्टीत शिबिरासाठी घेऊन गेला असताना त्यांना परोपकाराचे धडे देण्याची संधी त्याला मिळते.
मात्र त्यातून जो अनावस्था प्रसंग उद्भवतो आणि त्यात चिमणराव स्वत:च कसा अडकतो, याचे वर्णन चिमणराव स्वत: करतो. ‘धेनोयाघ्रः पलायते।’ या कथेत चिमणरावांच्या स्वभाववैशिष्ट्यांमुळे जी विनोदनिर्मिती होते त्यामुळे वाचकांच्या चेहऱ्यावर स्मितहास्य उमटते. त्याचप्रमाणे ही कथा म्हणजे एका प्रादेशिक भाषेतून प्राचीन भाषेतील अनुवादाचा उत्कृष्ट नमुना आहे.
The title ‘धेनोर्व्याघ्रः पलायते।’ is based on a famous समस्या in Sanskrit literature, ‘मृगात् सिंहः पलायते।’ which comes from the stories of king 11. In both the quotes, the author has imagined a situation exactly opposite to the nature’s law. These situations surely bring smile on the reader’s face.
This story is a translated version of a Marathi story ‘कॅप्टन चिमणराव – स्काउटमास्तर’ from a renowned story book ‘चिमणरावांचे चव्हाट’ and tuns published in annual संस्कृत magazine named ‘समर्पणम्’. चिमणराव, the hero of this story and an immortal character of Marathi literature, wished to train a troop of brave youngsters/teenagers, similar to the scouts.
His aim behind this training uns to inculcate qualities like benevolence, bravery, social service etc. in the minds of the young children. But as he took them for a camp during mcations and got an opportunity to teach them a lesson of benevolence, he himself got stuck in a difficult situation.
The story ‘धेनोर्व्याघ्रः पलायते।’ brings smile on the faces of the readers due to the humor caused due to innocent nature of चिमणराव. It is also an excellent example of translation skills from a regional language to the ancient language of India.

परिच्छेदः 1
शिबिरस्य प्रथमदिबसे ……… साहाय्यं करोतु।
अनुवादः
शिबिराच्या पहिल्या दिवशीच दुपारी जेवण वगैरे आवरून मी मुलांसोबत पत्ते खेळण्यात मग्न होतो. तेव्हा नुकतीच ओळख झालेले प्राध्यापक सर्कसवाले (सर्कशीचे मालक) मलाच शोधत तेथे पोहचले. “कॅप्टनसाहेब, ऐका कॅप्टनसाहेब! आमच्या सर्कशीत कित्येक महिन्यांपासून लोकप्रिय असा सहभोजनाचा कार्यक्रम चालतो.
गाय, अस्वल आणि वाघ इ. प्राण्यांचा हा कार्यक्रम प्रयोगाचे सर्वोच्च आकर्षण आहे. तसेच जाहिरात करुन तिकिटे सुद्धा विकली आहेत. परंतु वाघ आणि अस्वलामध्ये झालेल्या भांडणामुळे दोघेही जखमी झाले आहेत.”
“काय? प्राण्यांचे भांडण?” “साहेब, आमच्या सर्कशीच्या खेळात वाघ आणि अस्वल दोन पायांचे (मनुष्य) आहेत.” अशाप्रकारे प्राध्यापकमहोदयांनी रहस्यभेद केला. “अस्वल किंवा वाघ पाळण्याची आमची क्षमता नाही. दोन बहुरूपी पुरुषच प्रयोगात वाघ आणि अस्वलाचा अभिनय करतात.”
“गाय सुद्धा……?”
“नाही, नाही, गाय खरी आहे.”
“हं….. मग मी इथे काय करू सांगा?”
“मी विनंती करतो, की आपल्या बालवीरांच्या चमूतील कोणत्याही दोन सैनिकांनी जर आजच्या प्रयोगात वाघ-अस्वलाचा वेश घेऊन प्रेक्षकांचे मनोरंजन केले, तर खूप उपकार होतील. कृपया मदत करा.”
On the first day of the camp itself, I was engrossed in playing the game of cards with children, after completing lunch etc. in the afternoon At that time, professor सर्कसवाले (owner of a circus) who was newly introduced, reached there looking for me only.
“Respected captain, listen (to me). Our circus has a popular programme of a joint meal since many months. This programme of the cow, the bear and the tiger etc. animals is the biggest attraction of our show. Tickets are also sold with the advertisement. but due to a fight between the bear and the tiger, both are wounded.” “What? Fight between animals?” “Sir, the tiger and the bear in our circus are human indeed.”
Thus, the respected professor revealed the secret. “We are not able to pet a bear or a tiger. Two disguised men only enact the tiger and the bear in the show.” “Is the cow too….?” “No, No, the cow is real.” “Hmm…. tell me then what should I do here?” “I request that, if any two cadets from your troop of brave boys entertain the audience, disguised as the tiger and the bear in today’s show, it would be a great favour. Please help me.”

परिच्छेदः 2
सर्कसस्वामिनः विवशता ………….. क्षिपन्ति पाषाणखण्डान्।
अनुवादः
सर्कशीच्या मालकाची विवशता/अगतिकता माझ्या लक्षात आली. ही परोपकाराची संधी योगायोगाने मिळाली आहे, असा विचार करून त्याची मदत करावी असे मी ठरवले. अस्वलाच्या वेशात अब्दुल शोभेल अशा विचाराने त्याला अस्वलाचे पात्र दिले. वाघाचा अभिनय करण्यासाठी मी स्वत:च तयार झालो. अशाप्रकारे पात्रयोजना झाली.
कार्यक्रमापूर्वीच मी तसेच अब्दुल, वाघ आणि अस्वलाचा वेश घेऊन आपापल्या पिंजऱ्यात थांबलो. माझ्या पिंजऱ्यासमोरच जास्त गर्दी झाली होती. मी शक्यतोवर लोकांचे मनोरंजन करण्याचा प्रयत्न केला. परंतु लोक संतुष्ट नव्हते. “हा काय वाघ आहे ? (किती) दुबळा आहे,” असे थट्टेने म्हणत होते.
तेव्हाच एक बाई वाघ पाहण्यासाठी स्वत:च्या मुलासोबत आली. माझ्या गर्जनेमुळे मुलगा घाबरला, म्हणून ती आई माझ्यावर दगड फेकू लागली. तेव्हा वेदनेने व्याकुळ झालेला मी, वाघ बोलत नाही हे क्षणभर विसरून म्हणालो, “पिंजऱ्यातल्या वाघाला बघून महिला दगड फेकतात/ मारतात.”
I understood the helplessness of the owner of the circus. Thinking that, I have obtained an opportunity to help him by chance I decided to help him. I assigned the character of the bear to Abdul, thinking that he would be apt/suitable for the role. I myself got ready to enact the tiger.
Thus, the characters/roles were decided/fixed. Me and Abdul stayed in our own cages disguised as the tiger and the bear before the show itself. There was more crowd gathered in front of my cage. I tried to entertain people as much as possible but crowd was not satisfied. “Is this a tiger? How weak he is!” They said mockingly.
Then, a woman, along with her son came to see the tiger. The boy got afraid due to my roaring. Hence, that mother threw pieces of stones at me. At that time, for a moment I forgot that the tiger doesn’t speak; I said, “Seeing the caged tigers, women throw pieces of stones.”

परिच्छेदः 3
मम वाक्यसमाप्तेः ……… अभ्यस्ता आसीत्।
अनुवादः
माझं वाक्य संपण्यापूर्वीच, वाघाच्या माणसाच्या भाषेत बोलण्यामुळे मूर्च्छित झालेली ती महिला, ‘हे भूत आहे भूत….’ असे मोठ्याने किंचाळत जमिनीवर पडली. माझा मुलगा राघूसुद्धा एकदा पिंजऱ्याजवळ येऊन, “अप्पा, चहा प्यायचा आहे का?” असे विचारू लागला तेव्हा प्रेक्षक गोंधळात पडले. रात्री दहा वाजता सर्कशीचा खेळ सुरु झाला. बारा वाजता खेळाचा सर्वोच्च बिंदू आला – सहभोजन. प्रेक्षकांची उत्सुकता शिगेला पोहोचली.
गड्याने रंगमंचावर तीन खुा ठेवल्या व मधोमध गोलाकार टेबल ठेवला. एका खुर्चीत मला बसवले होते. नंतर अस्वलाच्या वेशातील अब्दुल तेथे आला. गाय सुद्धा मंचावर आली. त्यानंतर आमच्यासमोर खाद्यपदार्थ ठेवले गेले. सहभोजन सुरु होताच उत्तेजित झालेल्या प्रेक्षकांनी आनंदाने टाळ्या वाजवण्यास सुरुवात केली. पूर्वीसुद्धा वाघ आणि अस्वल ही माणसेच होती. परंतु ती गाय त्यांच्यासोबत कार्यक्रम करण्यासाठी सरावलेली होती.
Even before my sentence would end, that woman screaming aloud, “ghost, it’s a ghost”, fell on the ground being unconscious due to the tiger speaking human language. Even when my son coming near the cage asked, “Father, would you like to have some tea?”, the audience got confused. The circus show began at 100clock in the night. The peak point of the show came at 120dock- joint meal. The excitement of the audience reached the height.
A servant placed three chairs on the stage and a circular stool in the middle. I was made to sit in one chair. Then , disguised as a bear came there. Even the cow arrived on the stage. Then, food was arranged in front of us. As the meal started, the audience that was excited started clapping happily. Even earlier, the tiger and the bear were human. But the cow was trained/used to perform with them.

परिच्छेद: 4
अचिरादेव न इमौ ……… विभूषितानि आसन्।
अनुवादः
थोड्याच वेळात गायीच्या लक्षात आले की, हे वाघ आणि अस्वल | ओळखीचे नाहीत, ती गोंधळाने व संशयाने आम्हाला एकेक करून पाहू लागली.
परिस्थितीची जाणीव होताच तिने शिंग उगारून आमच्यावर हल्ला केला. मी सुद्धा गर्जना करुन माझे तीक्ष्ण दात दाखवले. पण ती जराही घाबरली नाही.
अब्दुलने हाताने तिला मारले. त्यामुळे चिडून ती त्याच्यामागे धावू लागली. अस्वल लगेच तंबूच्या खांबावर चढले. हा सगळा खेळ पूर्वनियोजित आहे असा विचार करुन प्रेक्षकसुद्धा खूश झाले. अस्वल निसटले म्हणून चिडलेली ती गाय आता माझ्याकडे – वाघाकडे वळली. भीतीमुळे चार पायाच्या वाघाचे रूप विसरून मी मूळच्या द्विपाद स्वरूपात आलो.
“प्राध्यापक महोदय, वाचवा, वाचवा”, असा करुण विलाप करत धावत सुटलो. तेव्हा, गाईला घाबरुन पळणाऱ्या वाघाला पाहून प्रेक्षकवर्गात प्रचंड गोंधळ उडाला. प्रेक्षकांनी माझा वाघाचा वेश काढून मला निर्दयीपणे मारले. वेदनेमुळे मी मूच्छित झालो आणि जेव्हा जाग आली तेव्हा आमची गाडी पुन्हा पुण्याच्या दिशेने निघाली होती. माझे मस्तक व इतर अवयव मलमपट्टीमुळे सुशोभित झालेले होते.
In a short while, the cow realized that these tiger and bear are not familiar. She looked at us turn by turn with confusion and doubt. When she realized the fact, she attacked us having raised her horns. I too having roared displayed sharp teeth. But she wasn’t even a little afraid.
अब्दुल beat her with hand. Being angry due to this, she ran after him. The bear quickly climbed the pole of the tent. The audience too got happy thinking that all this show is pre-planned. The cow being angry as the bear escaped, now targeted me, the tiger.
Forgetting the disguise of four-legged tiger due to fear, I resorted to my original bipedal form (stood up). I started running, lamenting “Respected professor, help, help.” At that time, seeing the tiger running, being afraid of the cow, huge chaos took place in the audience.
The audience beat me cruelly removing my tiger’s disguise. I fainted due to pain and when I woke up, our vehicle was already on the way back to Pune. My head and other organs were decorated with bandages.

शब्दार्थाः
- रहस्यभेदः – revelation of a secret – रहस्याचा उलगडा
- व्रणिती – (both) got wounded – (दोघे) जखमी झाले
- द्विपादौ – having two legs – दोन पायांचे प्राणी (दोन माणसे)
- बहुरूपधारिणौ – taking many forms – बहुरूपी
- पत्रक्रीडा – game of cards – पत्त्यांचा खेळ
- विज्ञापनम् – advertisement जाहिरात
- कलहकारणात् – due to quarrel/fight – भांडणामुळे
- मध्याहे – in the afternoon – दुपारी
- अन्विष्य – looking for/searching – शोधत शोधत
- पालयितुम् – to nurture – पाळण्यासाठी
- सम्मर्दः – crowd – गर्दी
- अवसर: – opportunity – संधी
- कृशः – weak/lean – दुबळा/क्षीण
- नियोजितः – appointed – नियुक्त केलेले
- बाधितः – affected – व्याकुळ झाला
- सिद्धः – got ready – तयार झाला
- विवशता – helplessness – अगतिकता
- अवगता – understood – समजली
- उपहासेन – mockingly – थट्टेने
- गर्जनया – due to roaring – गर्जनेमुळे
- स्वपञ्जरयो: – in own cages – स्वत:च्या पिंजऱ्यात
- अक्षिपत् – threw – फेकला
- रञ्जयितुम् – to entertain – मनोरंजन करण्यासाठी
- यदृच्छया – by chance – योगायोगाने
- पात्रयोजना – characters – पात्रयोजना
- निर्धारिता – were decided – निश्चित झाली
- चरमबिन्दुः – peak point – सर्वोच्च बिंदू
- उत्कण्ठा – excitement – उत्सुकता
- मूर्छिता – fainted – चक्कर/भोवळ आली
- आक्रोशन्ती – crying/shouting – ओरडत/किंचाळत
- तालिकावादनम् – clapping – टाळ्या वाजवणे
- मनुष्यवाण्या – in human speech – माणसाच्या भाषेत
- मझे – on stage – रंगमंचावर
- अभ्यस्ता आसीत् – was trained – प्रशिक्षित होती
- पराकाष्ठा जाता – reached height – शिखर गाठले
- कोलाहल: – chaos – गाँधळ
- शकटः – cart – गाडी/वाहन
- ताडितवान् – beat – मारले
- आरूद्धवान् – climbed – चढला
- विलपन् – lamenting – विलाप करीत
- क्षुब्धा/कुद्धा – angry – संतप्त
- उत्तमाङ्गम् – head – मस्तक
- प्रमुदिताः – delighted / happy – आनंदी
- अनुत्तमानि – lower – वाईट
- पटमण्डप स्तम्भम् – pole of the tent – तंबूचा खांब
- सम्भ्रमेण – due to confusion – गोंधळामुळे
- उपचारपट्टिकाभिः – with bandages – औषधाच्या पट्टीमुळे
- शृङ्गे – horns – शिंगे
- परित्रायताम् – help/save – वाचवा
- उन्नम्य – having raised – उगारून
- निष्कास्य – removing – काढून
- निघृणम् without mercy – दयामाया न दाखवता
Aamod Sanskrit Book 10th Class Solutions
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]()