Std 11 Hindi Chapter 10 Mahatvakansha Aur Lobh Question Answer Maharashtra Board
Balbharti Maharashtra State Board Hindi Yuvakbharati 11th Digest Chapter 10 महत्त्वाकांक्षा और लोभ Notes, Textbook Exercise Important Questions and Answers.
Hindi Yuvakbharati 11th Digest Chapter 10 महत्त्वाकांक्षा और लोभ Questions And Answers
11th Hindi Digest Chapter 10 महत्त्वाकांक्षा और लोभ Textbook Questions and Answers
आकलन
1. लिखिए :
प्रश्न अ.
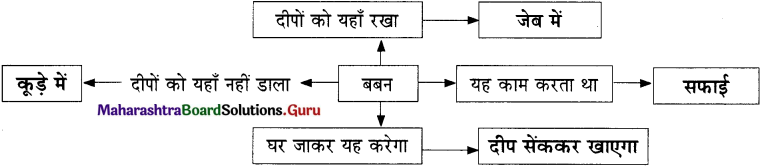
मछुवा-मछुवी की दिनचर्या –
……………………………………………………..
……………………………………………………..
उत्तर :
मछुवा दिन भर मछलियाँ पकड़ता।
मछुवी दिन भर दूसरा काम करती।
![]()
प्रश्न आ.
मछुवा-मछुवी की कहानी का अंत –
……………………………………………………..
……………………………………………………..
उत्तर :
मछली रूष्ट हो गई।
मछुवा-मछुवी का राजवैभव छिन लिया गया।
दोनों फिर से अपनी टूटी-फूटी झोपड़ी में रहने लगे।
प्रश्न इ.
लेखक द्वारा बताई गईं मनुष्य स्वभाव की विशेषताएँ –
(1) ……………………………………………………..
(2) ……………………………………………………..
(3) ……………………………………………………..
उत्तर :
(1) अपने दोषों को छिपाकर दूसरों पर दोषारोपण करना।
(2) अपने ही कामों को महत्त्व देना, दूसरों के नहीं।
(3) अपने द्वारा किए गए उपकार को निस्संकोच बताना परंतु दूसरों के द्वारा की गई सेवा को न बतलाना।
शब्द संपदा
2. निम्नलिखित शब्दों के लिए उचित शब्द समूह का चयन कीजिए :
(1) अभक्ष्य : जो खाने के अयोग्य हो / जो खाया नहीं गया।
उत्तर :
जो खाने के अयोग्य हो।
(2) अदृश्य : जो दिखाई न दे / जो दिखाई नहीं देता।
उत्तर :
जो दिखाई न दे।
(3) अजेय : जिसे जीता न जा सके / जिसे जितना कठिन हो
उत्तर :
जिसे जीता न जा सके।
(4) शोषित : जिसका शोषण किया गया है जो शोषण करता है।
उत्तर :
जिसका शोषण किया गया है।
(5) कृशकाय : जिसका शरीर कुश के समान हो / जो बहुत दुबला-पतला हो।
उत्तर :
जिसका शरीर कुश के समान हो।
![]()
(6) सर्वज्ञ : जो सब कुछ जानता हो / जो सब जगह व्याप्त है।
उत्तर :
जो सब कुछ जानता हो।
(7) समदर्शी : जो सबको समान देखता है / जो सबको समान दृष्टि से देखता है।
उत्तर :
जो सबको समान दृष्टि से देखता है।
(8) मितभाषी : जो कम बोलता है / जो मीठा बोलता है।
उत्तर :
जो कम बोलता है।
अभिव्यक्ति
3.
प्रश्न अ.
‘अति से तो अमृत भी जहर बन जाता है, इस कथन पर अपने विचार प्रकट कीजिए।
उत्तर :
‘अति का भला न बोलना, अति की भली न चूप।
अति का भला न बरसना, अति की भली न धूप।।’
अर्थात ‘अति’ हर जगह नुकसानदायी ही है। अति लालसा मनुष्य के जीवन में पतन के द्वार खोल देती है। कुछ पाने की आशा में वह अपना सब कुछ गँवा देता है। अपने नैतिक मूल्यों को अनदेखा कर मनुष्य सारी मर्यादाएँ तोड़कर इच्छापूर्ति में लग जाता है। पाठ में दी गई कहानी के मछुवा-मछुवी की तरह ही जो वैभव मिला था उसे फिर से गँवा बैठते हैं।
दुनिया गवाह है प्रकृति के साथ हमने जो ‘अति किया और कर रहे हैं उसका परिणाम आज प्रदूषण के रूप में भुगत रहे हैं। गुड़ के एक छोटे से टुकड़े का सेवन और स्वाद अच्छा होता है परंतु इस छोटे टुकड़े को बड़े टुकड़े में बदलकर उसका सेवन करने से शरीर में विकार ही उत्पन्न होंगे।
एक गुब्बारे में उसकी क्षमता से अधिक हवा भरने की कोशिश की तो परिणाम क्या होगा कहने की आवश्यकता नहीं है। जो दवा उचित अनुपान से ली गई तो अमृत के समान हमारी सेहत ठीक करती है वही दवा अगर अधिक मात्रा में ली गई तो उसके दुष्परिणाम जान लेवा ही सिद्ध होंगे।
अमृत भी जहर बन जाएगा यह बात त्रिकालाबाधित सत्य है। अत: ‘अति सर्वत्र वर्जयेत’ ध्यान में रखना है और ‘अति’ से बचना चाहिए।
![]()
प्रश्न आ.
‘महत्त्वाकांक्षाओं का कभी अंत नहीं होता’, इस वास्तविकता को अपने शब्दों में स्पष्ट कीजिए।
उत्तर :
प्रगति के लिए महत्त्वाकांक्षी होना उचित है परंतु इनका कोई अंत नहीं है। एक के पूरा होते ही दूसरी महत्त्वाकांक्षा जन्म ले लेती है। इच्छा, कामना, लालसा, महत्त्वाकांक्षा ये सभी तृष्णा के पर्यायवाची शब्द हैं। इन्हीं कारणों से मनुष्य नैतिक या अनैतिक मार्ग से भी क्यों न हो उसे पूरा करने में लग जाता है।
सपने देखना एक सहज प्रवृत्ति है परंतु उन्हें साकार न होते देख तनावग्रस्त होना गलत है। क्योंकि हम दूसरों के आधार पर अपना आकलन करने लगते हैं। दूसरों से आगे निकलना ही हमारे लिए महत्त्वपूर्ण बन जाता है। फिर वह खेल-कूद हो, पढ़ाई-लिखाई हो, घर-गृहस्थी हो या अन्य कुछ।
विचार, ज्ञान, पैसा, प्रतिष्ठा, बल, बुद्धि आदि में दूसरों से श्रेष्ठ बनने की महत्त्वाकांक्षा हममें जागती ही रहती है। वह हमें लोभ के माया जाल में फँसाती रहती है। उदाहरणार्थ – एक छोटा सा घर बनाने की महत्त्वाकांक्षा से जब अपना घर बनता है।
तब हमारे घर के सामने किसी का बड़ा घर बनते ही हमें अपने घर का आनंद होने की बजाय सामने वाले घर के समान अपना घर नहीं इस बात का दुःख होता है और हम उसी प्रकार के घर को बनाने की महत्त्वाकांक्षा में लग जाते हैं।
कितना भी मिला, कितना भी पाया तो भी मनुष्य संतुष्ट नहीं होता; महत्त्वाकांक्षा कभी समाप्त नहीं होती। यही जीवन की वास्तविकता है।
पाठ पर आधारित लघूत्तरी प्रश्न।
4.
प्रश्न अ.
प्रस्तुत निबंध में निहित मानवीय भावों से संबंधित विचार लिखिए।
उत्तर :
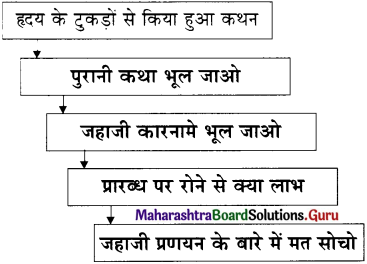
‘महत्त्वाकांक्षा और लोभ’ इस निबंध में लेखक श्री. पदुमलाल बख्शी जी ने महत्त्वाकांक्षा के साथ-साथ असंतोष, अति लालसा, स्वयं को शक्तिमान बनाने की उत्कट अभिलाषा तथा कृतघ्नता के दुष्परिणाम को प्रस्तुत किया है। जो सुलभता से प्राप्त होता है उसके प्रति अर्थात प्राप्य के प्रति विरक्ति का भाव तथा अप्राप्य की लालसा मनुष्य को किस तरह लोभ में फँसाती है इसे कहानी द्वारा स्पष्ट किया है।
मछुवा और मछुवी को मछली के वरदान से घर, धन, राजकीय वैभव प्राप्ति के साथ-साथ मछुवी रानी बनी और सेवा में नौकर-चाकर भी प्राप्त हुए। इतना सब-कुछ प्राप्त होने से जो मिला है, उससे संतुष्ट होना चाहिए था। पर मानवीय प्रवृत्ति ऐसी है कि जो कभी संतुष्ट रहने नहीं देती, जो अप्राप्य है उसे पाने का प्रयास करती रहती है। अति महत्त्वाकांक्षा ने सूर्य, चंद्र, मेघ को अपने वश में करने की लालसा ने उनका जीवन समाप्त किया।
मानवीय भावों को अपने वश में रखना सही है पर हम उसे अपने वश में रख नहीं पाते यही हकीकत है।
![]()
प्रश्न आ.
पाठ के आधार पर कृतघ्नता, असंतोष के संबंध में लेखक की धारणा लिखिए।
उत्तर :
पाठ की कहानी में देवी मछली की कृतघ्नता लेखक ने स्पष्ट की है। मछुवे ने देवी मछली की मदद निस्वार्थ भाव से की थी।
पत्नी के कहने पर उसने कुछ याचना की थी और उसे पूरा करके देवी मछली ने मछुए की पत्नी में अभिलाषा पैदा की थी। परंतु उसके सामने मछुवे ने पत्नी की ऐसी इच्छा प्रकट की थी जो वह पूरा नहीं कर सकती थी तब उसने सारा वैभव, धन सबकुछ वापस ले लिया और गरीबी में रहने का शाप दे दिया।
वरदान का अंत इस प्रकार अभिशाप में परिणत हो गया। देवी होते हुए भी उसमें त्याग, प्रेम, कृतज्ञता, क्षमा, दया जैसी भावनाएँ नहीं थी। वह कृतघ्न थी जो एक देवी को शोभा नहीं देता।
मछुवे की पत्नी में जो असंतोष था वह मानवी स्वभाव है। क्योंकि जब तक मनोवांछित फल मिलता नहीं तब तक उसे पाने के लिए मन लालायित रहता है परंतु जब वह वस्तु प्राप्त हो जाती है तब हमें दूसरी उससे भी बड़ी और महत्त्वपूर्ण वस्तु प्राप्त करने की इच्छा जाग जाती है और असंतोष की भावना मन में बनी रहती है। मछली ने पहले घर माँगा था। फिर खाने-पीने की तकलीफ है इसलिए धन माँगा था। लालसा बढ़ जाने पर राज वैभव माँगा था।
उसके पास महल, बाग, नौकर-चाकर आ जाने पर उसने सूर्य, चंद्र, मेघ आदि पर हुक्म करने की इच्छा व्यक्त की थी। अति लालसा और असंतोष के कारण ही जो कुछ उसने पाया था उसे खोना पड़ा था।
जो मछुवा-मछुवी वर्तमान में संतोष से जी रहे थे, टूटी-फूटी झोपड़ी में भी संतुष्ट थे उनमें असंतोष के भाव पैदा होने के कारण ही राज-वैभव भी उन्हें संतुष्ट नहीं कर पाया। मन की अनंत इच्छाओं का परिणाम ऐसा ही भयानक होता है यही लेखक का कहना है।
साहित्य संबंधी सामान्य ज्ञान
5. जानकारी दीजिए:
प्रश्न अ.
पदुमलाल पुन्नालाल बख्शी जी के निबंधों की प्रमुख विशेषताएँ –
(१) …………………………………………..
(२) …………………………………………..
उत्तर :
(1) कहानी जैसी मनोरंजकता
(2) जीवन की सच्चाइयों की बड़ी सरलता से अभिव्यक्ति
प्रश्न आ.
अन्य निबंधकारों के नाम –
उत्तर :
- राँगेय राघव
- रामधारीसिंह ‘दिनकर’
- हजारीप्रसाद द्विवेदी
- गुणाकर मुळे
- रवींद्रनाथ त्यागी
![]()
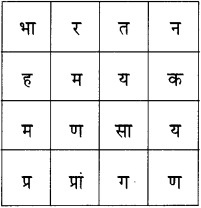
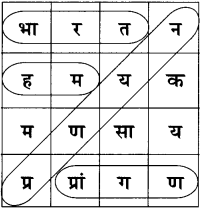
6. दी गई शब्द पहेली से सुप्रसिद्ध रचनाकारों के नाम ढूंढकर उनकी सूची तैयार कीजिए :
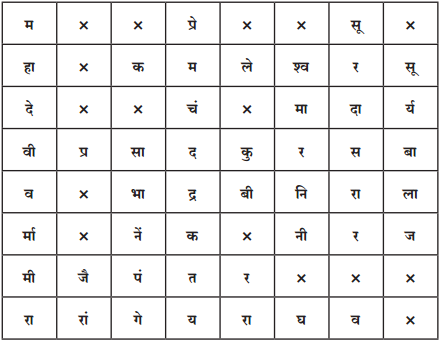
उत्तर :
- महादेवी वर्मा
- मीरा
- रांगेय राघव
- पंत (सुमित्रानंदन)
- कमलेश्वर
- प्रेमचंद
- निराला (सूर्यकांत त्रिपाठी)
- नीरज (गोपालदास सक्सेना)
- सूरदास
- प्रसाद (हरिशंकर)
- जैनेंद्र कुमार
Yuvakbharati Hindi 11th Textbook Solutions Chapter 10 महत्त्वाकांक्षा और लोभ Additional Important Questions and Answers
कृतिपत्रिका
(अ) निम्नलिखित गद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| गद्यांश : पर एक दिन एक घटना हो गई …………………………………. मछली उन्हें खाकर उसपर और भी प्रसन्न होती। (पाठ्यपुस्तक पृष्ठ क्र. 50-51) |
प्रश्न 1.
कारण लिखिए :
(i) मछली ने मछुवे को पुकारा –
(1) ………………………………..
(2) ………………………………..
उत्तर :
(1) नदी के किनारे लता में मछली फँसी थी।
(2) मछली अपने जीवनदान के लिए पुकार रही थी।
![]()
(ii) मछली को आनंद हुआ –
(1) ………………………………..
(2) ………………………………..
उत्तर :
(1) मछुवे ने मछली को पानी में छोड़ा।
(2) आटे की गोलियाँ खिलाई।
प्रश्न 2.
उत्तर लिखिए :
(i) मछली यहाँ फँसी थी – ………………………………..
(ii) नदी के पास था – ………………………………..
(iii) मछलियाँ पकड़ने आया था – ………………………………..
(iv) यहाँ खूब पानी था – ………………………………..
उत्तर :
(i) लताओं में
(ii) एक गड्ढा
(iii) मछुवा
(iv) नदी में
प्रश्न 3.
(i) विलोम शब्द लिखिए :
(1) तैरना : ………………………………
उत्तर :
डूबना
(ii) सुरक्षित शब्द सुरक्षा + इत अर्थात ‘इत’ प्रत्यय लगाकर वना है। ‘इत’ प्रत्यययुक्त अन्य शब्द लिखिए:
(1) …………………………………
(2) …………………………………
उत्तर :
(1) शिक्षा + इत = शिक्षित
(2) सीमा + इत = सीमित
प्रश्न 4.
अभिलाषा पूर्ति के आनंद को अपने अनुभव द्वारा व्यक्त कीजिए।
उत्तर :
हर मनुष्य को सुख की अभिलाषा रहती है। सुख-दुख का संबंध मनुष्य के शरीर से होता है जबकि आनंद का संबंध उसकी आत्मा से होता है। हम जो भी कर्म करते हैं फल की आशा हमें होती ही है और मनोनुकूल फल पाकर हमारा मन आनंदित हो उठता है।
प्रकृति के सौंदर्य का रसपान मेरे लिए सबसे बड़ा आनंद है। दैनंदिन जीवन की चिंताओं से दूर प्रकृति की गोद में बैठकर मौज-मस्ती करने में जो आनंद है वह शायद ही किसी अन्य साधन से मिलता होगा। कामकाज की थकान क्षण में काफूर हो जाती है।
ऋषि-मुनियों की आध्यात्मिक चेतना यहाँ जागृत होती रही और हमारी संस्कृति का विकास हुआ। कवियों के अंत:स्थल से काव्य की अभिव्यक्ति हुई। एक कवि ने क्या खूब लिखा है –
‘पर्वतों की श्रृंखलाओं में ये कौन सा जादू है छिपा,
ऐसा लगा मुझे जीवन का सबसे हँसीन पल मिला।’
![]()
(आ) निम्नलिखित गद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| गद्यांश : मछुवा नदी के तट पर ………………………………… मछली से यही माँगो। (पाठ्यपुस्तक पृष्ठ क्र. 51) |
प्रश्न 1.
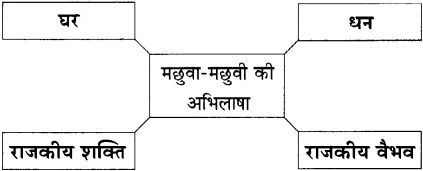
संजाल पूर्ण कीजिए :

उत्तर :
प्रश्न 2.
कारण लिखिए :
(i) घर होने से लाभ नहीं हुआ क्योंकि …………………………………
उत्तर :
घर होने से लाभ नहीं हुआ क्योंकि खाने-पीने की तकलीफ थी।
(ii) धन प्राप्त होने से लाभ नहीं हुआ क्योंकि …………………………………
उत्तर :
धन प्राप्त होने से लाभ नहीं हुआ क्योंकि मछवी को राजवैभव चाहिए था।
प्रश्न 3.
निम्नलिखित शब्दों को उपसर्ग लगाकर सही शब्द वनाओ।
(i) दिन :
(ii) घर
(ii) धन
(iv) एक
उत्तर :
(i) दुर्दिन
(ii) बेघर
(iii) निर्धन
(iv) अनेक.
![]()
प्रश्न 4.
‘लालच बुरी वात है’ इस विषय पर अपने विचार लिखिए।
उत्तर :
जब कभी इंसान लालच करता है वह अपना ही नुकसान कर बैठता है। लालच के कारण हमारी संपत्ति, रिश्ते-नाते सब बिगड़ जाते हैं। लालच में पड़कर एक भाई अपने भाई को, पति-पत्नी एक दूसरे को धोखा देते हैं। इतना ही नहीं तो कोई गद्दार अपनी मातृभूमि को भी धोखा दे सकता है।
ऐसा करने वाले सभी अंत में स्वयं का ही नुकसान कर बैठते हैं। लालच के चलते गलत काम करके मुसीबत में फंसने वाले कितने ही लोग हमें आस-पास ही देखने मिल जाएँगे। लालच इंसान को इंसान नहीं रहने देती। लालच ऐसी बुरी बला है कि हमें सफलता के रास्ते से दूर ले जाती है।
सत्तालिप्सा, धनलोलुपता, पदलोलुपता के चलते मनुष्य मानवता को भी ताक पर रख देता है। इतिहास इसका गवाह है। अत: लालच से हमेशा दूर रहकर नैतिक पतन से बचना चाहिए और सफलता की ओर अग्रसर होना चाहिए।
(इ) निम्नलिखित गद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
| गद्यांश : यदि मैं मछुवा होता तो ……………………………………….. उपकार की भावना नहीं है, क्षमा नहीं, दया नहीं है। (पाठ्यपुस्तक पृष्ठ क्र. 52) |
प्रश्न 1.
संजाल पूर्ण कीजिए :

उत्तर :

प्रश्न 2.
परिणाम लिखिए :
(i) मछुवी को मछली की दैवी शक्ति पर विश्वास हो जाने का परिणाम –
उत्तर :
मछुवी को मछली की दैवी शक्ति पर विश्वास हो जाने का परिणाम यह हुआ कि मछुवी ने ऐसी इच्छा प्रकट कर दी जो पूरी करना असंभव था।
(ii) देवी समझकर याचना करने का परिणाम –
उत्तर :
देवी समझकर याचना करने का परिणाम यह हुआ कि मछुवे की पत्नी के मन में अभिलाषाएँ पैदा हुई।
![]()
प्रश्न 3.
पर्यायवाची शब्द लिखिए :
(i) हृदय : ……………………………………………..
(ii) विश्वास : ……………………………………………..
उत्तर :
(i) हिय, उर, दिल
(ii) यकीन, आस्था, भरोसा
अभिव्यक्ति :
प्रश्न 1.
‘अति से तो अमृत भी जहर वन जाता है’ इस कथन पर अपने विचार प्रकट कीजिए।
उत्तर :
संत कबीर कहते है,
‘अति का भला न बोलना, अति की भली न चूप।
अति का भला न बरसना, अति की भली न धूप।।’
अर्थात ‘अति’ हर जगह नुकसानदायी ही है। अति लालसा मनुष्य के जीवन में पतन के द्वार खोल देती है। कुछ पाने की आशा में वह अपना सब कुछ गँवा देता है। अपने नैतिक मूल्यों को अनदेखा कर मनुष्य सारी मर्यादाएँ तोड़कर इच्छापूर्ति में लग जाता है। पाठ में दी गई कहानी के मछुवा-मछुवी की तरह ही जो वैभव मिला था उसे फिर से गँवा बैठते हैं।
दुनिया गवाह है प्रकृति के साथ हमने जो ‘अति किया और कर रहे हैं उसका परिणाम आज प्रदूषण के रूप में भुगत रहे हैं। गुड़ के एक छोटे से टुकड़े का सेवन और स्वाद अच्छा होता है परंतु इस छोटे टुकड़े को बड़े टुकड़े में बदलकर उसका सेवन करने से शरीर में विकार ही उत्पन्न होंगे।
एक गुब्बारे में उसकी क्षमता से अधिक हवा भरने की कोशिश की तो परिणाम क्या होगा कहने की आवश्यकता नहीं है। जो दवा उचित अनुपान से ली गई तो अमृत के समान हमारी सेहत ठीक करती है वही दवा अगर अधिक मात्रा में ली गई तो उसके दुष्परिणाम जान लेवा ही सिद्ध होंगे।
अमृत भी जहर बन जाएगा यह बात त्रिकालाबाधित सत्य है। अत: ‘अति सर्वत्र वर्ज येत’ ध्यान में रखना है और ‘अति’ से बचना चाहिए।
महत्त्वाकांक्षा और लोभ Summary in Hindi
महत्त्वाकांक्षा और लोभ लेखक परिचय :
पदुमलाल पुन्नालाल बख्शी जी का जन्म 27 मई 1894 को खैरागढ़ (छत्तीसगढ़) में हुआ। शिक्षा के उपरांत आप साहित्य के क्षेत्र में आए। साहित्य क्षेत्र में आपकी निबंध, उपन्यास तथा समीक्षात्मक ग्रंथों में अलग पहचान दिखाई देती है। जीवन के कठीन सिद्धांत अर्थात तत्वों को दृष्टांत के सहारे स्पष्ट करने की आपकी शैली अद्वितीय है। आपका साहित्य समाज का दर्पण (mirror) ही नहीं बल्कि दीपक है।
जीवन की सच्चाइयों को बड़ी सरलता से व्यक्त करना तथा कहानी-सी मनोरंजकता के साथ प्रस्तुति आपके साहित्य की विशेष शैली बनी है। साहित्य और समाज सेवा में आपका जीवन बीता और 1971 में आपने इस संसार से बिदा ली।
![]()
महत्त्वाकांक्षा और लोभ प्रमुख कृतियाँ :
‘कथा चक्र’ (उपन्यास), “हिंदी साहित्य विमर्श’ और ‘विश्व साहित्य’ (समीक्षात्मक ग्रंथ), बख्शी ग्रंथावली, ‘पंचपात्र’, ‘पद्यवन’, ‘कुछ’, और कुछ (निबंध संग्रह)
महत्त्वाकांक्षा और लोभ विधा का परिचय :
‘निबंध’ एक गद्य विधा है। किसी विषय का यथार्थ चित्रण जिसमें किया जाता है। निबंध इस गद्य विधा से जीवन के तत्वों को बड़ी सरलता के साथ समाज के सामने रखा जाता है। वर्तमान परिस्थितियों का काफी सूक्ष्म चित्रण निबंध जैसी विधा में किया जाता है।
आचार्य हजारीप्रसाद द्विवेदी, आचार्य रामचंद्र शुक्ल, कन्हैयालाल मिश्र ‘प्रभाकर’ आदि निबंधकारों ने इस विधा को उच्च कोटी पर पहुँचा दिया है।
महत्त्वाकांक्षा और लोभ विषय प्रवेश :
ज्ञात से अज्ञात की ओर इसी शिक्षा प्रणाली की तरह प्रस्तुत निबंध में जीवन के तत्वों को आरंभ में काल्पनिक कथा से जोड़ दिया है। मछुवा और मछुवी की काल्पनिक कहानी हमें सरलता से समझा देती है कि, जीवन की अति महत्त्वाकांक्षा, अति लालसा, सर्वशक्तिमान होने की अभिलाषा जीवन को परास्त (defeated) करती है।
महत्त्वाकांक्षा और लोभ परिणामत:
मछुवा-मछुवी का सामान्य जीवन, मछली का वरदान, अभिलाषाओं का जागृत होना, मानवीय भावों को वश में न रखना, वरदान शाप में परिणत होना – मानवीय भावों के इस खेल में क्या सही, क्या गलत, दोष मछली का या मछुवी का – यही निबंध के चिंतन विषय हैं।
महत्त्वाकांक्षा और लोभ पाठ परिचय :
‘अति से तो अमृत भी जहर बन जाता है’ जीवन के इसी तथ्य को उजागर करने वाले इस निबंध में अति महत्त्वाकांक्षा के साथ असंतोष, अति लालसा, लोभ, स्वयं को सर्वशक्तिमान बना लेने की उत्कट अभिलाषा जीवन को परास्त कर देती है।
जो मिला है, जितना मिला है, उसी में संतुष्ट रहने के बजाय अधिक पाने की अभिलाषा मनुष्य को लोभ के जाल में फँसाती है। मछली के वरदान से मछुवा-मछुवी को घर मिला, धन मिला, राजकीय वैभव मिलने से मछुवी रानी भी बनी।
पर हिरण्यकश्यप की तरह सर्वशक्तिमान होने की अभिलाषा से उन्होंने सूर्य, चंद्र, तथा मेघ को अपनी आज्ञा में रहने का वरदान माँगा। मछली अप्रसन्न होकर शाप देती है – ‘जा-जा, अपनी उसी झोपड़ी में रह।’ वरदान शाप में परिणत होते ही मछुवा-मछुवी झोंपड़ी में रहने लगे।
यहाँ एक तरफ अभिलाषा है। अभिलाषाओं को जगाने वाली मछली है। मानवीय भावों के इस खेल में दोष किसका? यही तो निबंध का सार है।
![]()
महत्त्वाकांक्षा और लोभ पाठ का सारांश :
अप्राप्य की लालसा हमेशा मानव मन को लोभ के जाल में फँसाती रहती है और जीवन को तहस-नहस कर डालती है। जीवन के इसी सिद्धांत को इस निबंध में दृष्टांत द्वारा समझाया है।
एक कछुवा और कछुवी अपनी टूटी-फूटी झोंपड़ी में अपना जीवन व्यतीत कर रहे थे। मछुवा दिनभर मछलियाँ पकड़ता तो मछुवी दिन भर दूसरा काम करती थी तब कहीं खाने को मिलता था। यही उनका वर्तमान था, उन्हें न आशा थी, न कोई लालसा।
मछुवा एक दिन मछली पकड़ने नदी के किनारे गया। वहाँ नदी के किनारे एक छोटी सी मछली लताओं में फँसी थी। मछली ने मछुवे को देखकर पुकारा और मदद माँगी कि, मुझे पानी में छोड़ दो। मछुवे ने निस्वार्थ भाव से मछली को पानी में छोड़ा।
मछली ने पहले गड्ढे के पानी में, फिर नदी के पानी में छोड़ने की बात की। मछुवे ने वैसा ही किया। फिर मछली ने मछुवे को नदी के किनारे रोज आकर बैठने की बात की ताकि उसका मन बहल जाए। मछुवा वैसा ही करता रहा।
पत्नी के पूछने पर मछुवे ने पूरी घटना बता दी। पत्नी ने कहा तुम कुछ नहीं समझते, वह मछली कोई साधारण नहीं है। मछली के रूप में कोई देवी होगी। उससे कुछ माँग लो।

पत्नी के कहने पर मछुवे ने मछली से अपने लिए घर माँगा। मछली के वरदान से मछुवे का घर बन गया। मछुवी में लोभ जागा। उसने सोचा घर होने से क्या होगा? धन चाहिए। फिर उसने धन माँगा तो धन मिला पर मछुवी की महत्त्वाकांक्षा बढ़ गई। उसने राजवैभव माँगा। फिर राजवैभव मिल गया।
![]()
उसका लोभ बढ़ा और उसने फिर रानी होने की अभिलाषा रखी। मछुवी राजमहल में रानी बन गई। अति लोभ से मछुवी ने अपने पति से कहलवाकर मछली से – सूर्य, चंद्र, मेघ पर अपने अधिकार में होने की माँग की। मछली ने रुष्ट होकर कहा – “जा – जा अपनी उसी झोपड़ी में रह।”
मछली के इसी शाप से सब समाप्त होकर मछुवा और मछुवी अपनी उसी – टूटी-फूटी झोंपड़ी मे आ गए। कथा समाप्त हो गई।
प्रस्तुत निबंध से लेखक बताना चाहते हैं कि मछुवा और मछुवी की कही कथा सच नहीं थी पर लोगों के मनोरथों की कथा सच है।
महत्त्वाकांक्षा और लोभ शब्दार्थ :
- रुष्ट = अप्रसन्न, नाराज
- मनोरथ = इच्छा, कामना
- व्यग्रता = अधीरता
- परिणत = रूपांतरित
- रुष्ट = अप्रसन्न, नाराज (angry),
- मनोरथ = इच्छा, कामना (desire),
- व्यग्रता = अधीरता, बैचेनी (anxiety),
- निर्बुद्धि = अल्पमति, अज्ञानी, नासमझी (ignorance),
- अप्राप्य = जो प्राप्त नहीं (inaccessible),
- परिणत = रूपांतरित (converting)
Hindi Yuvakbharati 11th Digest Maharashtra Board