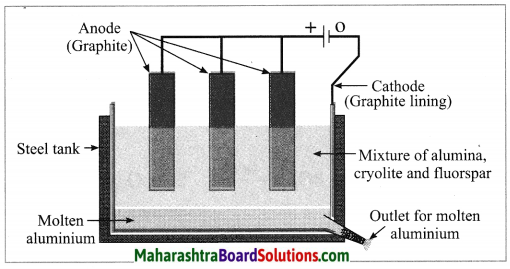
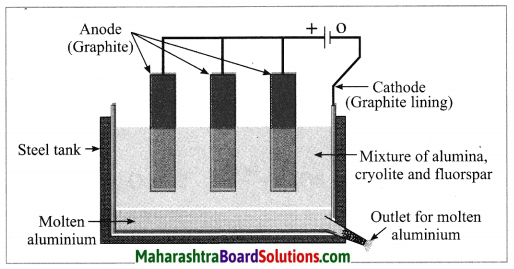
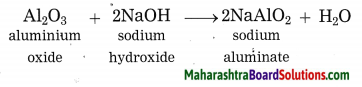
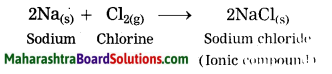
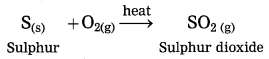
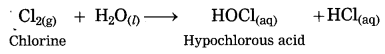
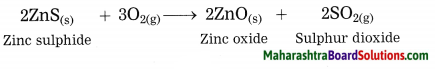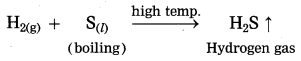Balbharti Maharashtra State Board Class 10 Science Solutions Part 1 Chapter 9 Carbon Compounds Notes, Textbook Exercise Important Questions and Answers.
Std 10 Science Part 1 Chapter 9 Carbon Compounds Question Answer Maharashtra Board
Class 10 Science Part 1 Chapter 9 Carbon Compounds Question Answer Maharashtra Board
Question 1.
Match the pairs.
| Group A | Group B |
| a. C2H6 | 1. Unsaturated hydrocarbon |
| b. C2H2 | 2. Molecular formula of an alcohol |
| c. CH4O | 3. Saturated hydrocarbon |
| d. C3H6 | 4. Triple bond |
Question 2.
Draw an electron dot structure of the following molecules. (Without showing the circles)
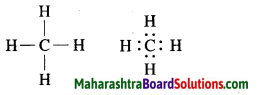
a. Methane.
Answer:
Molecular formula: CH4
b. Ethene.
Answer:
Molecular formula: H2C = CH2

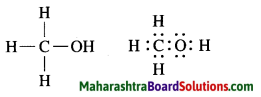
c. Methanol.
Answer:
Molecular formula: H3C – OH
d. Water.
Answer:
Molecular formula: H2O
![]()
![]()
Question 3.
Draw all possible structural formulae of compounds from their molecular formula given below.
a. C3H8
b. C4H10
c. C3H4
Answer:
a. C3H8 Propane:
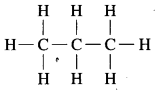
b. C4H10 Butane:
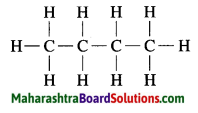
c. C3H4 Propyne:
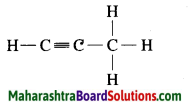
Question 4.
Explain the following terms with example.
a. Structural isomerism.
Answer:
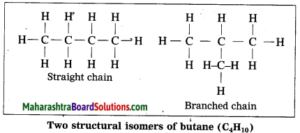
The phenomenon in which compounds having different structural formulae have the same molecular formula is called structural isomerism. Butane is represented by two different compounds as their structural formulae are different. The first compound is a straight chain compound and the second compound is a branched chain compound. These two different structural formulae have the same molecular formula i.e. C4H10.
b. Covalent bond.
Answer:
The chemical bond formed by sharing of two valence electrons between the two atoms is called covalent bond.
Example:
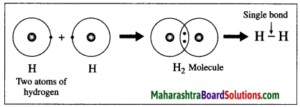
1. Hydrogen molecule formation: The atomic number of hydrogen is 1, its atom contains 1 electron in K shell. It requires one more electron to complete the K shell and attain the configuration of helium (He). To meet this requirement two hydrogen atoms share their electrons with each other to form H2 molecule. One covalent bond, i.e. a single bond is formed between two hydrogen atoms by sharing of two electrons.
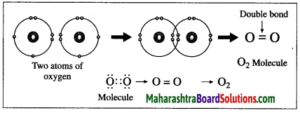
2. Formation of oxygen molecule:
(1) The atomic number of oxygen is 8. The electronic configuration of oxygen is (2, 6). Oxygen has 6 electrons in the outermost shell.
(2) It requires 2 electrons to complete the L shell and attain the configuration of neon (Ne).
(3) Each oxygen atom shares its valence electron with the valence electron of another oxygen atom to give two shared pairs of electrons which results in the formation of oxygen molecule.
(4) Thus, two electron pairs are shared between two oxygen atoms, forming double covalent bond (=).
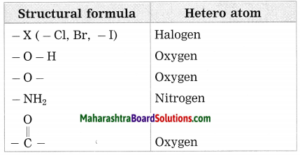
c. Hetero atom in a carbon compound.
Answer:
Carbon compounds are formed by formation of bonds of carbon with other elements such as halogens, oxygen, nitrogen, sulfur. The atoms of these elements substitute one or more hydrogen atoms in the hydrocarbon chain and thereby the tetravalency of carbon is satisfied. The atom of the element which is substitute for hydrogen is referred to as a heteroatom. Sometimes hetero atoms are not alone but exist in the form of certain groups of atoms.
![]()
d. Functional group.
Answer:
The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of length and nature of the carbon chain in that compound. Therefore these hetero atoms or groups of atoms containing hetero atoms are called the functional groups.
e. Alkane.
Answer:
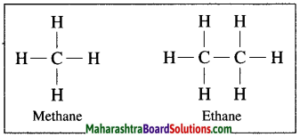
In hydrocarbon, the four valencies of carbon atom are satisfied only by the single bonds, such compounds are called alkane.
Example: In methane, four hydrogen atoms are bonded to carbon atom by four single covalent bonds.
f. Unsaturated hydrocarbons.
Answer:
The carbon compounds having a double bond or triple bond between two carbon atoms are called unsaturated hydrocarbons. The unsaturated hydrocarbons containing a carbon-carbon double bond are called alkenes.
e.g. Ethene (CH2 = CH2), Propene (CH3 – CH = CH2).
The unsaturated hydrocarbons containing a carbon-carbon triple bond are called alkynes e.g. Ethyne (CH ≡ CH).
g. Homopolymer.
Answer:
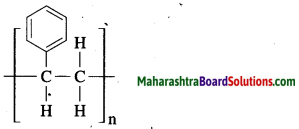
The polymers formed by repetition of single monomer are called homopolymer. e.g. polyethylene (CH2 – CH2)n.
h. Monomer.
Answer:
The small unit that repeats regularly to form a polymer is called monomer.
Example: Ethylene.
i. Reduction.
Answer:
In a chemical reaction, removal of oxygen from a compound or addition of hydrogen to a compound is called a reduction.
j. Oxidant.
Answer:
An oxidant is a reactant that oxidizes or removes electrons from other reactants during a redox reaction. An oxidant may also be called an oxidizer or oxidizing agent. When the oxidant includes oxygen, it may be called an oxygenation reagent or oxygen-atom transfer (OT) agent.
Examples of oxidants include:
- Hydrogen peroxide
- Ozone
- Nitric acid
- Sulfuric acid
![]()
Question 5.
Write the IUPAC names of the following structural formulae.
a. CH3 – CH2 – CH2 – CH3
Answer:
The number of carbon atopic in the longest chain: 4
Parent alkane: Butane IUPAC name: n-Butane
b. CH3 – CHOH – CH3 (Practice Activity Sheet – 3)
Answer:
The number of carbon atoms in the longest chain: 3
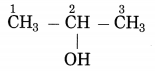
Parent alkane: Propane
Functional group: -OH (ol)
Assign the number: 2
The carbon atom to which the -OH group is attached is numbered as C2. If the carbon chain of the compound contains a -OH group then change the ending of the parent name, i.e., ‘e’ of propane is replaced by ‘ol’. (ol stands for alcohol)
Parent suffix: Propan-2-ol
IUPAC name: Propan-2-ol
c. CH3 – CH2 – COOH (Practice Activity Sheet – 3)
Answer:
The number of carbon atoms in the longest chain: 3
Parent alkane: Propane
Functional group: -COOH (-oic acid)
If the carbon chain of the compound contains a -COOH group then change the ending of the parent name, i.e., ‘e’ of propane is replaced by ‘oic acid’.
Parent suffix: Propanoic acid
IUPAC name: Propanoic acid
d. CH3 – CH2 – NH2
Answer:
Number of carbon atoms: 2
Parent alkane: Ethane
Functional group: -NH2 (amine)
If the carbon chain of the compound contains a -NH2 group, then change the ending of the parent name, i.e., ‘e’ of ethane is replaced by ‘amine’.
Parent suffix: Ethanamine
IUPAC name: Ethanamine.
e. CH3 – CHO
Answer:
Number of carbon atoms: 2
Parent alkane: Ethane
Functional group: -CHO (al)
If the carbon chain of the compound contains a -CHO group, then change the ending of the parent name, i.e., ‘e’ of ethane is replaced by ‘al’.
Parent suffix: Ethanal
IUPAC name: Ethanal
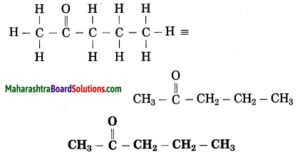
f. CH3 – CO – CH2 – CH3
Answer:
Number of carbon atoms in the longest chain: 4
Parent alkane: Butane Functional group: -CO- (one)
Assign the number:
![]()
In the longest chain, the numbering of carbon atom starts from the carbon atom nearest to the function group.
If the carbon chain of the compound contains a (-CO-) group, then change the ending of the parent name, i.e., ‘e’ of butane is replaced by ‘one’.
Parent suffix: Butan-2-one
IUPAC name: Butan-2-one
![]()
Question 6.
Identify the type of the following reaction of carbon compounds.
1. CH3 – CH2 – CH2 – OH + (O) → CH3 – CH2 – COOH
2. CH3 – CH2 – CH3 + O2 → 3CO2 + 4H2O
3. CH3 – CH = CH – CH3 + Br2 → CH3 – CHBr – CHBr – CH3
4. CH3 – CH3 + Cl2 → CH3 – CH2 – Cl + HCl
5. CH3 – CH2 – CH2 – CH2 – OH → CH3 – CH2 – CH = CH2 + H2O
6. CH3 – CH2 – COOH + NaOH → CH3 – CH2 – COONa+ + H2O
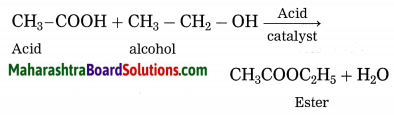
7. CH3 – COOH + CH3 – OH → CH3 – COO – CH3 + H2O
Answer:
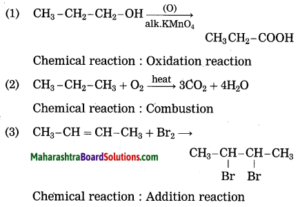
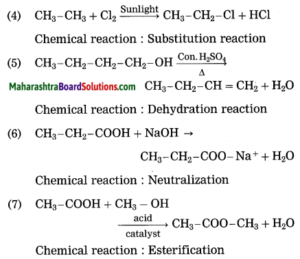
Question 7.
Write the structural formulae for the following IUPAC names:
a. Pent-2-one
Answer:
Pent-2-one.
(1) Pent stands for 5 carbon atoms in a chain.
Number the carbon atoms in a chain as 1, 2, 3,…..
![]()
(2) ‘one’ stands for functional group
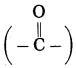
ketone. The number assigned for the ketone group is 2. Show the ketone group at C2.
(3) Now satisfy the valencies of each carbon atom
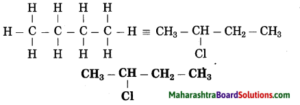
b. 2-Chlorobutane
Answer:
(1) In 2-chlorobutane, butane is parent alkane stands for 4 carbon atoms and number the carbon atoms in a chain as 1, 2, 3,….
![]()
(2) Chloro (Halo) is the prefix and the number assigned for prefix (chloro) is 2. Show the chloro atom at C2
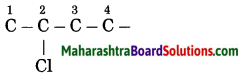
(3) Now satisfy the valencies of each carbon atom
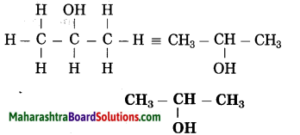
c. Propan-2-ol
Answer:
(1) Propan stands for 3 carbon atoms in a chain. Number the carbon atom in a chain as 1, 2, 3.
![]()
(2) ‘-ol’ stands for (-OH) hydroxyl group. The number assigned for the hydroxyl group is 2. Show the -OH group at C2.

(3) Now satisfy the valencies of each carbon atom
d. Methanal
Answer:
(1) Meth – stands for one carbon atom and assigned the number ‘1’ to carbon in the functional group -CHO.
(2) ‘-al’ stands for functional group (-CHO) aldehyde.
(3) Now satisfy the valencies of carbon in -CHO.
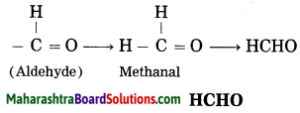
e. Butanoic acid
Answer:
(1) But stands for 4 carbon atoms in a chain. Number the carbon atoms in a chain as 1, 2, 3,….
![]()
‘-oic acid’ stands for functional group -COOH. Assign the number 1 to carbon in the functional group -COOH.
![]()
Now satisfy the valencies of each carbon atom
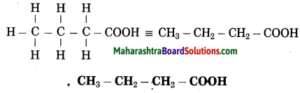
![]()
f. 1-Bromopropane.
Answer:
(1) In 1-bromopropane, propane is parent alkane stands for 3 carbon atoms and number the carbon atoms in a chain as 1, 2, 3…….
![]()
(2) Bromo (Halo) is the prefix and the number assigned for prefix (bromo) is 1, show the bromine atom at C1.
![]()
(3) Now satisfy the valencies of each carbon atom
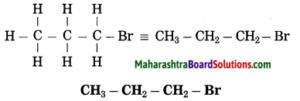
g. Ethanamine
Answer:
(1) Eth stands for 2 carbon atoms in a chain and the parent alkane is ethane.
– C – C –
(2) ‘amine’ stands for (- NH2) amino group. Show the amino (-NH2) at any carbon atom.
– C – C – NH2
(3) Now satisfy the valencies of each carbon atom
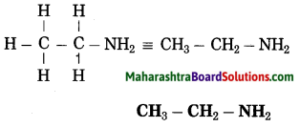
h. Butanone.
Answer:
(1) But stands for 4 carbon atoms in a chain and the parent alkane is butane. Number the carbon atoms in a chain 1, 2, 3,….
![]()
(2) ‘one’ stands for functional group
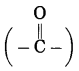
ketone. The number assigned for the ketone group is 2. Show the ketone group at C2.

(3) Now satisfy the valencies of each carbon atom
Question 8.
a. What causes the existance of very large number of carbon compound?
Answer:
(1) Carbon has a unique ability to form strong covalent bonds with other carbon atoms; this results in formation of big molecules. This property of carbon is called catenation power. The carbon compounds contain open chains or closed chains of carbon atoms. An open chain can be a straight chain or a branched chain. A closed chain is a ring structure. The covalent bond between two carbon atoms is strong and therefore stable. Carbon is bestowed with catenation power due to the strong and stable covalent bonds.
(2) One, two or three covalent bonds can bond together two carbon atoms. These bonds are called single covalent bond, double covalent bond and triple covalent bond respectively. Due to the ability of carbon atoms to form multiple bonds as well as single bonds, the number of carbon compounds increases. For example, there are three compounds, namely, ethane (CH3 – CH3), ethene (CH2 = CH2) and ethyne (CH = CH) which contain two carbon atoms.
(3) Carbon being tetravalent, one carbon atom can form bonds with four other atoms (carbon or any other). This results in formation of many compounds. These compounds possess different properties as per the atoms to which carbon is bonded. For example, five different compounds are formed using one carbon atom and two monovalent elements hydrogen and chlorine: CH4, CH3Cl, CH2Cl2, CHCl3, CCl4. Similarly carbon atoms form covalent bonds with atoms of elements like O, N, S, halogen and P to form different types of carbon compounds in large number.
(4) Isomerism is one more characteristic of carbon compound which is responsible for large number of carbon compounds.
![]()
b. Saturated hydrocarbons are classified into three types. Write these names giving one example each.
Answer:
In hydrocarbon, the four valencies of carbon atom are satisfied only by the single bonds, such compounds are called saturated hydrocarbons. Methane molecule contains only one carbon atom. In methane, four hydrogen atoms are bonded to carbon atom by four covalent bonds.

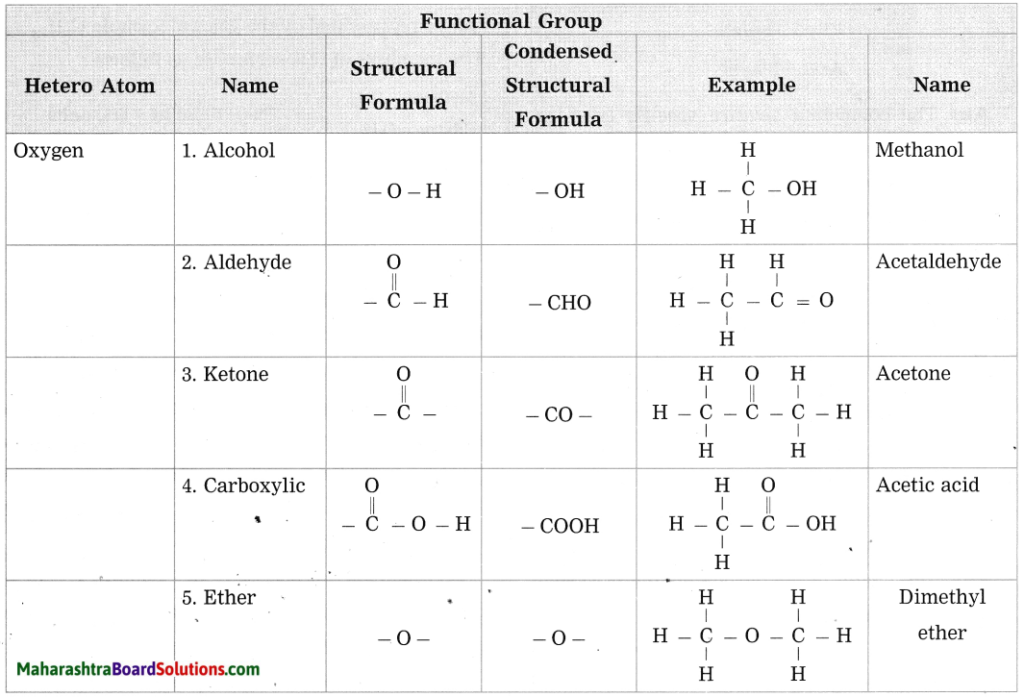
c. Give any four functional groups containing oxygen as the heteroatom in it. write name and structural formula of one example each.
Answer:
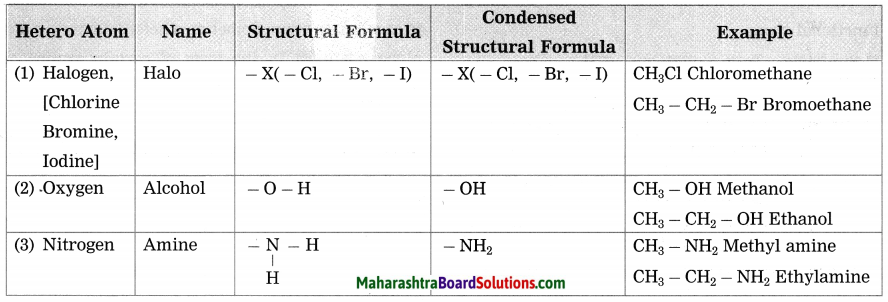
d. Give names of three functional groups containing three different heteroatoms. Write name and structural formula of one example each.
Answer:
e. Give names of three natural polymers. write the place of their occurance and names of monomers from which they are formed.
Answer:
- Poly saccharide is a natural polymer. It occurs in starch/carbohydrates. It is formed from monomer glucose.
- Protein is a natural polymer. It occurs in muscles, hair, enzymes, skin, egg. It is formed from alpha amino acids.
- Rubber is a natural polymer. It occurs in latex of rubber tree. It is formed from monomer isoprene.
f. What is meant by vinegar and gasohol? What are their uses?
Answer:
(1) Vinegar is a 5 – 8% aqueous solution of acetic acid. It is used as preservative in pickles. It is used to cook meat. 1t is used as a salad dressing.
(2) To increase the efficiency of petrol, it is mixed with 10% anhydrous ethanol, such a fuel is called gasohol. It is used as a fuel in cars and other vehicles.
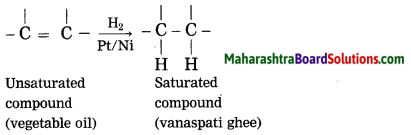
g. what is a catalyst ? write any one reaction which is brought about by use of catalyst?
Answer:
Catalyst is a substance, which changes the rate of reaction, without causing any disturbance to it. Vegetable oil (unsaturated compound) undergoes addition reaction with hydrogen in the presence of nickel catalyst to form vanaspati ghee (saturated compound).
Project:
Prepare a chart giving detailed information of carbon compounds in everyday use. Display it in the cluss and discuss.
![]()
Can you recall? (Text Book Page No. 110)
Question 1.
What are the types of compounds?
Answer:
Organic and inorganic compounds are the two important types of compounds.
Question 2.
Objects in everyday use such as foodstuff, fibres, paper, medicines, wood, fuels are made of various compounds. Which constituent elements are common in these compounds?
Answer:
The constituent elements common in these compounds are carbon (C), hydrogen (H) and oxygen (O).
Question 3.
To which group in the periodic table does the element carbon belongs? Write down the electronic configuration of carbon and deduce the valency of carbon.
Answer:
The element carbon belongs to group 14 and its electronic configuration is 2, 4. The valency of carbon is 4.
Use your brain Power! (Text Book Page No. 115)
Question 1.
The molecular formula of ethyne is C2H2. From this draw its structural formula and electron-dot structure.
Answer:
Ethyne: Molecular formula: C2H2
![]()
Question 2.
How many bonds have to be there in between the carbon atoms in ethyne so as to satisfy their tetra valency?
Answer:
To satisfy their tetravalency, three double bonds have to be there in between two carbon atoms in ethyne.
Use your brain power! (Text Book Page No. 116)
Question 1.
Draw the electron-dot structure of cyclohexane.
Answer:
Cyclohexane: Molecular formula: C6H12
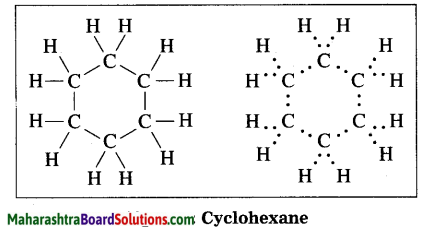
Use your brain power! (Text Book Page No. 112)
Question 1.
Atomic number of chlorine is 17. What is the number of electrons in the valence shell of the chlorine?
Answer:
There are seven electrons in the valence shell of the chlorine.
![]()
Question 2.
Molecular formula of chlorine is Cl2. Draw electron-dot and line structure of a chlorine molecule.
Answer:
![]()
Question 3.
The molecular formula of water is H2O. Draw electron-dot and line structures for triatomic molecule. (Use dots for electron of oxygen atom and crosses for electrons of hydrogen atoms.)
Answer:
![]()
Question 4.
The molecular formula of ammonia is NH3. Draw electron-dot and line structures for ammonia molecule.
Answer:
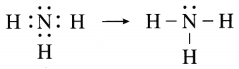
Question 5.
The molecular formula of carbon dioxide is CO2. Draw the electron-dot structure (without showing circle) and line structure for CO2.
Answer:
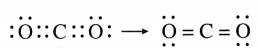
Question 6.
With which bond C atom in CO2 is bonded to each of the O atoms ?
Answer:
In CO2, carbon atom is bonded to each of the O atoms by double bond.
Question 7.
The molecular formula of sulfur is S8 in which eight sulphur atoms are bonded to each other to form one ring. Draw electron-dot structure for S8 without showing the circles.
Answer:
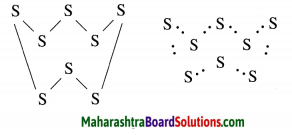
The above S8 molecule of sulphur has crown shaped structure. One molecule of sulpur is made up of eight atoms of sulphur.
Use your brain power! (Text Book Page No. 113)
Question 1.
Hydrogen peroxide decomposes of its own by the following reaction:
2H – O – O – H → 2H – O – H + O2
From this, what will be your inference about the strength O – O covalent bond ?
Tell from the above example whether oxygen has catenation power or not.
Answer:
In hydrogen peroxide (H2O2), the O – O covalent bond is not strong as oxygen has no catenation power.
| Name | Molecular formula |
Condensed Structural formula | Number of carbon atoms | Number of -CH2– units |
Boiling point ° C |
| Ethene | C2H4 | CH2 = CH2 | 2 | 0 | -102 |
| Propone | C3H6 | CH3 – CH = CH2 | 3 | 1 | -48 |
| 1-Butene | C4H8 | CH3 – CH2 – CH = CH2 | … | … | -6.5 |
| 1-Pentene | C5H10 | CH3 – CH2 – CH2 – CH = CH2 | … | … | 30 |
![]()
Use your brain power! (Text Book Page No. 120)
Question 1.
The above table shows the homologous series of alkenes. Inspect the molecular formulae of the members of this series. Do you find any relationship, in the number of carbon atoms and the number of hydrogen atoms in the molecular formulae?
Answer:
In the above homologous series, if we observe the molecular formulae of alkenes then the number of carbon atoms are half the number of hydrogen atoms.
Question 2.
If the number of carbon atoms in the molecular formulae of alkenes is denoted by ‘n’ what will be the number of hydrogen atoms?
Answer:
If the number of carbon atoms in the molecular formulae of alkenes is denoted by ‘n’ then the number of hydrogen atoms would be 2n.
Question 3.
What would be the general formula for the molecular formulae of the members of the homologous series of alkanes? What would be the value of ‘n’ for the first member of this series?
Answer:
The general formula for the homologous series of alkane is CnH2n + 2. The value of ‘n’ for the first member of homologous series is 1.
CnH2n+2 = C1H2 × 1 + 2 = CH4
Question 4.
The general molecular formula for the homologous series of alkynes is CnH2n – 2. Write down the individual molecular formulae of the first, second and third members by substituting the values 2, 3 and 4 respectively for ‘n’ in this formula.
Answer:
The general molecular formula for the homologous series of alkynes is CnH2n – 2
n = 2 C2H2 × 2 – 2 = C2H2 Ethyne
n = 3 C3H2 × 3 – 2 = C3H4 Propyne
n = 4 C4H2 × 4 – 2 = C4H6 Butyne
![]()
Question 5.
Write down structural formulae of the first four members of the various homologous series formed by making use of the functional groups.
Answer:
| Functional group Halo – X (Cl, Br, -I) | Functional group Aldehyde – CHO |
Functional group Carboxylic acid – COOH |
Functional group Amine -NH2 |
| CH3Cl Chloromethane |
HCHO Methanal |
HCOOH Methanoic acid |
CH3NH2 Methenamine |
| CH3 – CH2 – Cl Chloroethane |
CH3CHO Ethanal |
CH3COOH Ethanoic acid |
CH3CH2NH2 Ethanamine |
| CH3 – CH2 – CH2 – Cl 1-Chloropropane |
CH3CH2CHO Propanal |
CH3CH2COOH Propanoic acid |
CH3CH2CH2NH2 Propanamine |
| CH3 – CH2 – CH2 CH2 – Cl 1-Chlorobutane |
CH3CH2CH2 CHO Butanal |
CH3CH2CH2COOH Butanoic acid |
CH3CH2CH2CH2NH2 Butanamine |
Question 6.
General formula of the homologous series of alkanes is CnH2n + 2. Write down the molecular formula of the 8th and 12th member using this.
Answer:
General formula of alkanes is CnH2n + 2
n = 8 C8H2 × 8 + 2 = C6H18 Octane
n = 12 C12H2 × 12 + 2 = C12H26 Dodecane
Use your brain power! (Text Book Page No. 121)
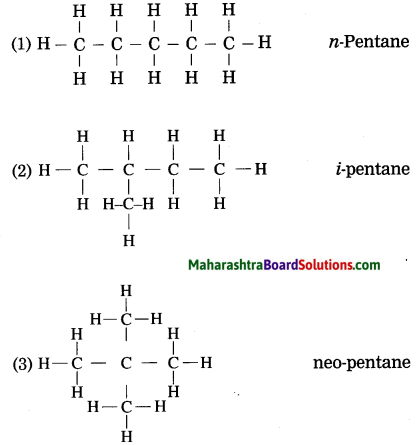
Question 1.
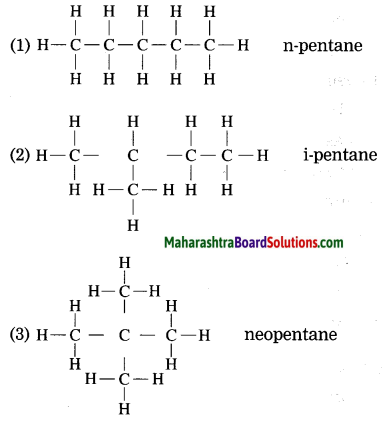
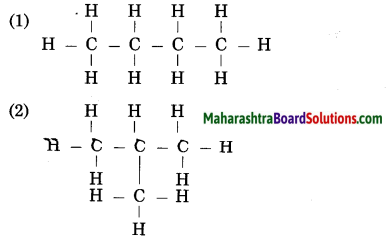
Draw three structural formulae having molecular formula C5H12. Give the names n-pentane, i-pentane and neo-pentane to the above structural formulae.
Answer:
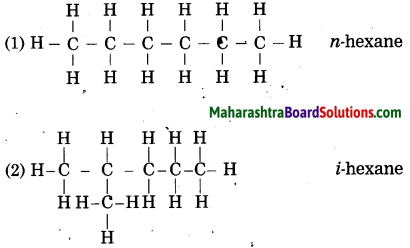
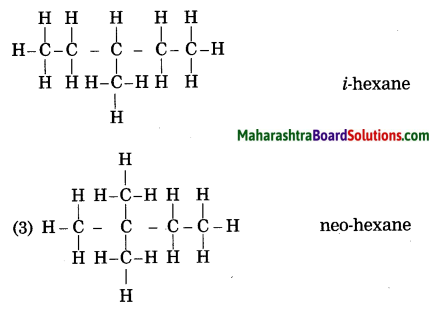
Question 2.
Draw all possible structural formulae having molecular formula C6H14. Give names to all the isomers.
Answer:
![]()
Try This! (Text Book Page No. 124)
Question 1.
Light a bunsen burner. Open and close the air hole at the bottom of the burner by means of the movable ring around it. When do you get yellow sooty flame? When do you get blue flame?
Answer:
When the air hole at the bottom of the burner is open, sufficient oxygen is mixed gaseous fuel for complete combustion and a clean blue flame is obtained. When the air hole is partially blocked by means of the movable ring around it, the air supply is limited which results in incomplete combustion. Hence, yellow sooty flame is produced.
(Text Book Page No. 126)
Question 1.
The names of four fatty acids separated from vegetable oils are given in the table. Identify the number of carbon – carbon double bonds from their structure and molecular formula from the below fatty acids which one when reacts with iodine will make the colour of iodine disappear.
Answer:
| Name | Molecular Formula | Number of C = C double bonds | Will it decolorise I2? |
| Stearic acid | C17H35COOH | ———————– | |
| Oleic acid | C17H33COOH | One double bond | yes/ |
| Plamitic acid | C15H31COOH | ———————– | |
| Linoleic acid | C17H31COOH | Two double bonds | yes/ |
Use your brain power! (Text Book Page No. 128)
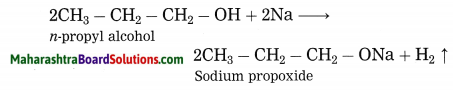
Question 1.
Explain by writing a reaction, what will happen when pieces of sodium metal are put in n-propyl alcohol.
Answer:
n-Propyl alcohol reacts with pieces of sodium metal, sodium propoxide and hydrogen gas are obtained.
Question 2.
Explain by writing a reaction, which product will be formed on heating n-butyl alcohol with concentrated sulphuric acid.
Answer:
When n-butyl alcohol is heated with concentrated sulphuric acid, one molecule of water is removed from its molecule to form 1-butene.
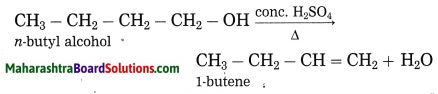
Use your brain power! (Text Book Page No. 129)
Question 1.
Which one of ethanoic acid and hydrochloric acid is stronger?
Answer:
Hydrochloric acid is stronger acid.
Question 2.
Which indicator paper out of blue litmus paper and pH paper is useful to distinguish between ethanoic acid and hydrochloric acid ?
Answer:
pH paper is useful to distinguish between ethanoic acid and hydrochloric acid.
![]()
Use your brain power! (Text Book Page No. 130)
Question 1.
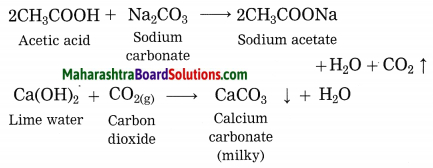
Explain why does the lime water turns milky in the reaction of acetic acid with sodium carbonate.
Answer:
In the reaction of acetic acid with sodium carbonate, carbon dioxide gas is evolved which turns lime water milky resulting in the formation of insoluble calcium carbonate.
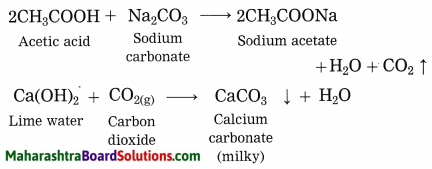
Question 2.
Explain the reaction that would take place when a piece of sodium metal is dropped in ethanoic acid.
Answer:
When a piece of sodium metal is dropped in ethanoic acid, sodium acetate and hydrogen gas is formed.
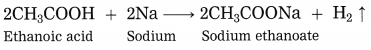
Question 3.
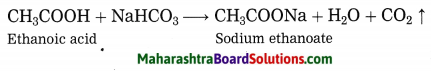
Two test tubes contain two colourless liquids ethanol and ethanoic acid. Explain by writing reaction which chemical test you would perform to tell which substance is present in which test tube.
Answer:
Ethanol does not react with sodium bicarbonate, while ethanoic acid reacts with sodium bicarbonate to form carbon dioxide gas.
Use your brain power! (Text Book Page No. 131)
Question 1.
When fat is heated with sodium hydroxide solution, soap and glycerin are formed. Which functional groups might be present in fat and glycerin? What do you think?
Answer:
The functional group carboxylic acid (-COOH) is present in fat whereas the functioned group hydroxyl group (-OH) is present in glycerin.
Can you tell? (Text Book Page No. 131)
Question 1.
What are the chemical names of the nutrients that we get from the foodstuff, namely, cereals, pulses and meat?
Answer:
The nutrients that we get from the foodstuff, namely cereals, pulses and meat are alpha amino acids.
Question 2.
What are the chemical substances that make cloth, furniture and elastic objects?
Answer:
The chemical substances that make cloth, furniture and elastic objects are cellulose and rubber.
Use your brain power! (Text Book Page No. 133)
Question 1.
Structural formulae of some monomers are given below. Write the structural formula of the homopolymer formed from them.
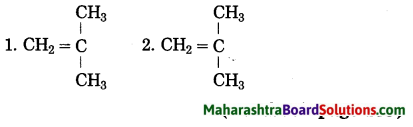
Answer:
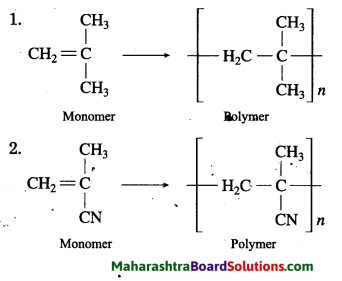
Question 2.
From the given structural formula of polyvinyl acetate, that is used in paints and glues, deduce the name and structural formula of the corresponding monomer.
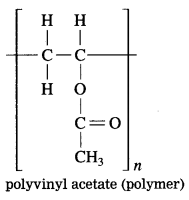
Answer:

(Text Book Page No. 133)
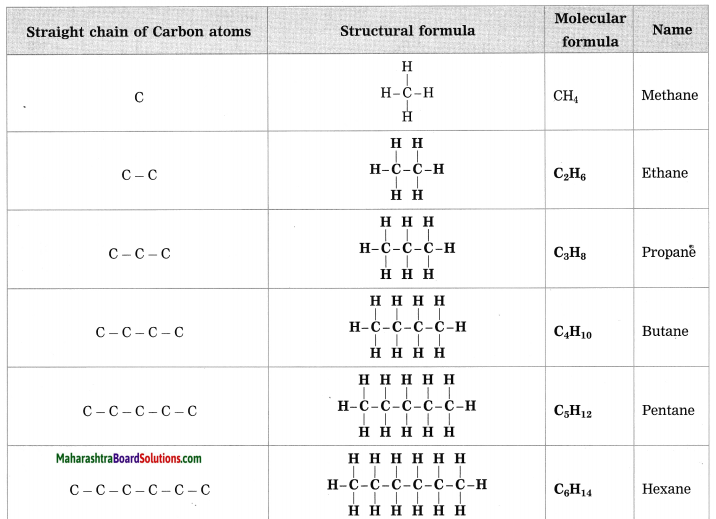
Question 1.
Complete the following table by writing their Structural formulae and Molecular formulae.
Answer:
(Answer is given directly in bold letters.)
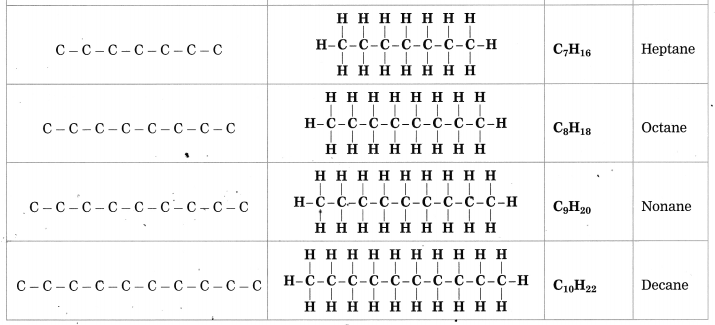
![]()
Fill in the gaps in the table: (Text Book Page No. 119)
(Answer is given directly in bold letters.)
a. Homologous series of alkanes.
Answer:
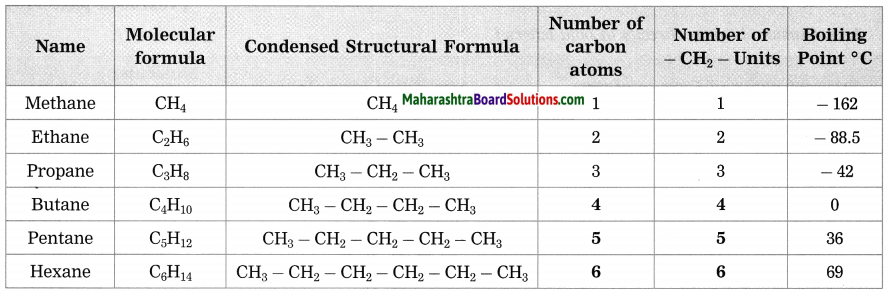
b. Homologous series of alcohol.
Answer:
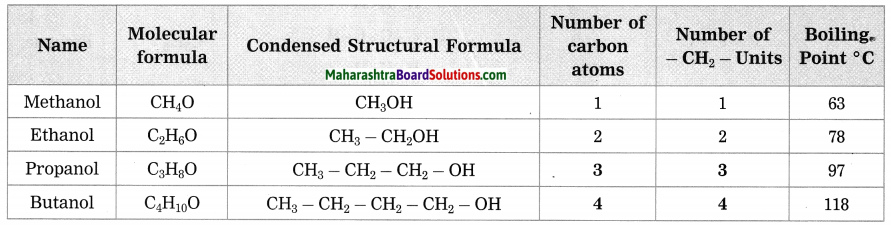
c. Homologous series of alkenes.
Answer:
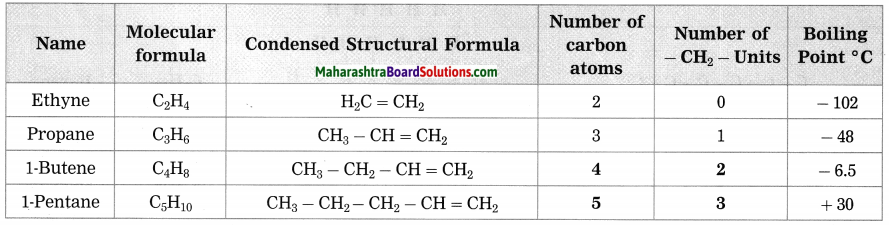
(Text Book Page No. 123)
Question 1.
Complete the table by writing the IUPAC names in the third column.
(Answer is directly given with underline.)
Answer:
| Common name | Structural formula | IUPAC name |
| Ethylene | CH2 = CH2 | Ethene |
| Acetylene | HC = CH | Ethyne |
| Acetic acid | CH3 -COOH | Ethanoic acid |
| Methyl alcohol | CH3 – OH | Methanol |
| Ethyl alcohol | CH3 – CH2 – OH | Ethanol |
Use your brain power! (Text Book Page No. 119)
Question 1.
By how many -CH2– (methylene) units do the formulae of the first two members of homologous series of alkanes, methane (CH4) and ethane (C2H6) differ? Similarly, by how many -CH2– units do the neighbouring members ethane (C2H6) and propane (C3H8) differ from each other?
Answer:
The first two members of homologous series of alkanes, methane (CH4) and ethane (C2H6) differed by one -CH2– unit. Similarly, ethane (C2H6) and propane (C3H8) differed by -CH2– unit.
Question 2.
How many methylene units are extra in the formula of the fourth member than the third member of the homologous series of alcohols?
Answer:
There is only one, methylene unit extra in the formula of the fourth member and the third member of the homologous series of alcohols.
Question 3.
How many methylene units are less in the formula of the second member than the third member of the homologous series of alkenes?
Answer:
There is only one methylene unit less in the formula of the second member of and the third member of the homologous series of alkenes.
![]()
Fill in the blanks and rewrite the completed statements:
Question 1.
The organic compounds having double or triple bonds in them are termed as …………
Answer:
The organic compounds having double or triple bonds in them are termed as unsaturated hydrocarbons.
Question 2.
The general formula of alkane is ……………
Answer:
The general formula of alkane is CnH2n + 2.
Question 3.
The compounds of homologous series have the same ………….. group.
Answer:
The compounds of homologous series have the same functional group.
Question 4.
A double bond is formed between carbon atoms by ………… pairs of electrons.
Answer:
A double bond is formed between carbon atoms by two pairs of electrons.
Question 5.
The compounds having different structural formulae having the same molecular formula is called ……….
Answer:
The compounds having different structural formulae having the same molecular formula is called structural isomerism.
Question 6.
The functional group of ether is …………..
Answer:
The functional group of ether is -O-.
Question 7.
The general formula of alkene is …………
Answer:
The general formula of alkene is CnH2n.
Question 8.
The bond between two atoms of nitrogen is a ………… bond.
Answer:
The bond between two atoms of nitrogen is a triple bond.
Question 9.
Benzene ring is made up of ………….. carbon atoms.
Answer:
Benzene ring is made up of six carbon atoms.
Question 10.
Due to …………., vegetable oil is converted into vanaspati ghee.
Answer:
Due to hydrogenation, vegetable oil is converted into vanatspati ghee.
Question 11.
………….. control the heredity at molecular level.
Answer:
Nucleic acids control the heredity at molecular level.
Question 12.
The regular repetition of a small unit is called …………..
Answer:
The regular repetition of a small unit is called polymer.
![]()
Question 13.
The structural formula of polypropylene is ……………….
Answer:
The structural formula of polypropylene is
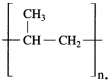
Question 14.
The monomers of proteins are ……………..
Answer:
The monomers of proteins are alpha amino acids.
Question 15.
The monomer of cellulose is …………
Answer:
The monomer of cellulose is glucose.
Question 16.
…………. have sweet odour.
Answer:
Esters have sweet odour.
Choose the correct alternative and rewrite the statement:
Question 1.
The property of direct bonding between atoms of the same element to form a chain is called ………..
(a) catenation
(b) isomerism
(c) dehydration
(d) polymerization
Answer:
The property of direct bonding between atoms of the same element to form a chain is called catenation.
Question 2.
The molecular weight of two adjacent members in homologous series of an alkane differ by ………. units.
(a) 16
(b) 20
(c) 14
(d) 12
Answer:
The molecular weight of two adjacent members in homologous series of an alkane differ by 14 units.
Question 3.
Consecutive members of a homologous series differ by ………. group.
(a) -CH
(b) -CH2
(C) -CH3
(d) -CH4
Answer:
Consecutive members of a homologous series differ by CH2 group.
Question 4.
……….. is used to prepare carbon black.
(a) Methane
(b) Ethene
(c) Propane
(d) Butane
Answer:
Methane is used to prepare carbon black.
Question 5.
……….. is the general formula of alkene.
(a) CnH2n
(b) CnH2n + 2
(c) CnH2n – 2
(d) CnHn – 2
Answer:
CnH2n is the general formula of alkene.
Question 6.
The reaction of methane with chlorine in the presence of sunlight is called ………..
(a) pyrolysis
(b) an elimination reaction
(c) a substitution reaction
(d) an addition reaction
Answer:
The reaction of methane with chlorine in the presence of sunlight is called a substitution reaction.
Question 7.
The general formula for alkynes is ………….
(a) CnH2n
(b) CnH2n + 2
(c) CnH2n – 2
(d) CnH2n – 1
Answer:
The general formula for alkynes is CnH2n – 2
Question 8.
The reaction of ………… with ethanol is a fast reaction.
(a) calcium
(b) magnesium
(c) sodium
(d) aluminum
Answer:
The reaction of sodium with ethanol is a fast reaction.
![]()
Question 9.
Ethylene has …………. bond between two carbon atoms.
(a) a single
(b) a double
(c) a triple
(d) an ionic
Answer:
Ethylene has a double bond between two carbon atoms.
Question 10.
The saturated hydrocarbons are those in which carbon atom are linked by ………….
(a) a single bond
(b) a double bond
(c) a triple bond
(d) an ionic bond
Answer:
The saturated hydrocarbons are those in which carbon atom are linked by a single bond.
Question 11.
C7H16 is ………….
(a) hexane
(b) octane
(c) methane
(d) heptane
Answer:
C7H16 is heptane.
Question 12.

(a) carboxylic acid group
(b) aldehyde group
(c) ketonic group
(d) alcohol group
Answer:
(a) carboxylic acid group
Question 13.
The possible isomers for C5H12 are ……………
(a) 2
(b) 4
(c) 1
(d) 3
Answer:
The possible isomers for C5H12 are 3.
Question 14.
………. contains alcoholic functional group.
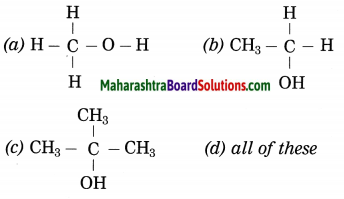
Answer:
(d) all of these
Question 15.
Oxygen molecule has ………… bond between two oxygen atoms.
(a) a double
(b) a single
(c) a triple
(d) an ionic
Answer:
Oxygen molecule has a double bond between two oxygen atoms.
Question 16.
Some acetic acid is treated with solid NaHCO3. The resulting solution will be ………..
(a) colourless
(b) blue
(c) green
(d) yellow
Answer:
Some acetic acid is treated with solid NaHCO3. The resulting solution will be colourless.
Question 17.
Ethanoic acid has a ……… odour.
(a) rotten eggs
(b) pungent
(c) mild
(d) vinegar-like
Answer:
Ethanoic acid has a vinegar-like odour.
![]()
Question 18.
Acetic acid ………..
(a) turns red litmus blue
(b) has pungent odour
(c) is red in colour
(d) is odourless
Answer:
Acetic acid has pungent odour.
Question 19.
When acetic acid reacts with sodium metal ……….. gas is formed.
(a) oxygen
(b) hydrogen
(c) chlorine
(d) nitrogen
Answer:
When acetic acid reacts with sodium metal hydrogen gas is formed.
Question 20.
The molecular formula of acetic acid (ethanoic acid) is …………
(a) HCOOH
(b) CH3COOH
(c) C2H5COOH
(d) C3H7COOH
Answer:
The molecular formula of acetic acid (ethanoic acid) is CH3COOH.
Question 21.
When sodium bicarbonate solution is added to dilute acetic acid …………
(a) a gas is evolved
(b) a solid settles at the bottom
(c) the mixture becomes warm
(d) the colour of the mixture becomes yellow
Answer:
When sodium bicarbonate solution is added to dilute acetic acid a gas-is evolved.
Question 22.
2 ml of ethanoic acid was taken in each of test tubes A, B, C and 2 ml, 4 ml, 6 ml of water was added respectively to them. A clear solution is obtained in ………..
(a) test tube A
(b) test tube B
(c) test tube C
(d) all the test tubes
Answer:
2 ml of ethanoic acid was taken in each of test tubes A, B, C and 2 ml, 4 ml, 6 ml of water was added respectively to them. A clear solution is obtained in all the test tubes.
Question 23.
In the presence of acid catalyst, ethanoic acid reacts with ethanol and ……….. ester is produced.
(a) ethanol
(b) ethanoic
(c) ethyl ethanoate
(d) ethyl ethanol (Practice Activity Sheet – 1)
Answer:
In the presence of acid catalyst, ethanoic acid reacts with ethanol and ethyl ethanoate ester is produced.
Question 24.
The following structural formula belongs to which carbon compound?
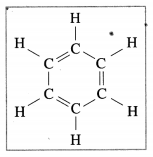
(a) Camphor
(b) Benzene
(c) Starch
(d) Glucose (Practical activity sheet- 2)
Answer:
(b) Benzene
Question 25.
What type of reaction is shown below?
\(\mathrm{CH}_{4}+\mathrm{Cl}_{2} \stackrel{\text { Sunlight }}{\longrightarrow} \mathrm{CH}_{3}-\mathrm{Cl}+\mathrm{HCl}\)
(a) Addition
(b) Substitution
(c) Decomposition
(d) Reduction (Practice Activity Sheet – 3)
Answer:
(b) substitution
Question 26.
The carbon compound is used in daily life is ………..
(a) edible oil
(b) salt
(c) carbon dioxide
(d) baking soda (March 2019)
Answer:
The carbon compound is used in daily life is edible oil
State whether the following statements are true or false. (If a statement is false, correct it and rewrite it.):
Question 1.
Generally the melting and boiling points of carbon compounds are high.
Answer:
False. (Generally the melting and boiling points of carbon compounds are low.)
Question 2.
Till now the number of known carbon compounds is about 10 million.
Answer:
True.
Question 3.
Unsaturated hydrocarbons are less reactive than saturated hydrocarbons.
Answer:
False. (Unsatured hydrocarbons are more reactive than saturated hydrocarbons.)
![]()
Question 4.
Benzene is an aromatic compound.
Answer:
True.
Question 5.
The carbon-carbon double and triple bonds are also recognised as functional groups.
Answer:
True.
Question 6.
The general formula of alkyne is CnH2n.
Answer:
False. (The general formula of alkyne is CnH2n – 2)
Question 7.
Naphthalene burns with a yellow flame.
Answer:
True.
Question 8.
When vegetable oil and tincture iodine react, the color of iodine does not change.
Answer:
False. (When vegetable oil and tincture iodine react, the colour of iodine changes.)
Question 9.
Saturated fats are healthy.
Answer:
False. (Saturated fats are harmful to health.)
Question 10.
Aqueous solution of ethanol is found to be neutral.
Answer:
True.
Question 11.
Denatured ethanol is used as industrial solvent.
Answer:
True.
Question 12.
Vinegar is a 12-15 % aqueous solution of acetic acid.
Answer:
False. (Vinegar is a 5-8 % aqueous solution of acetic acid.)
Question 13.
The functional group of ethanoic acid is a carboxylic group.
Answer:
True.
Question 14.
Sodium hydroxide is used in the preparation of soap from fats and oils.
Answer:
True.
Question 15.
Rubber is a manmade macromolecule.
Answer:
False. (Rubber is a natural macromolecule.)
Question 16.
Polyvinyl chloride is used in the manufacture of P.V.C. pipes and bags.
Answer:
True.
![]()
Question 17.
Polyethylene is a homopolymer.
Answer:
True.
Question 18.
The chemical bonds in carbon compounds do not produce ions.
Answer:
True.
Find the odd one out:
Question 1.
Propane, methane, ethene, pentane
Answer:
Ethene. (Others are saturated hydrocarbons.)
Question 2.
Methane, butane, benzene, sodium chloride
Answer:
Sodium chloride. (Others are organic compounds.)
Question 3.
CH4, C2H6, C3H8, CaCO3
Answer:
CaCO3. (Others are organic compounds.)
Question 4.
C2H2, C3H8, C2H6, CH4
Answer:
C2H2. (Others are saturated hydrocarbons.)
Question 5.
C2H4, C4H10, C3H8, CH4
Answer:
C2H4. (Others are saturated hydrocarbons.)
Question 6.
Polyethylene, Polysaccharide, Polystyrene, Polypropylene
Answer:
Polysaccharide (Others are manmade polymers.)
Question 7.
-NH2, -COOH,-SO4, -Br
Answer:
-SO4 (Others are functional groups.)
Question 8.
Methane, Ethane, Propene, Propane, Butane
Answer:
Propene (Others are members of homologous series of alkanes.)
Match the columns:
Question 1.
| Column I | Column II |
| (1) CH4 | (a) CH2 = CH2 |
| (2) Ethane | (b) CnH2n – 2 |
| (3) Alkene | (c) Methane |
| (4) Alkyne | (d) C2H6 |
| (e) C3H8 |
Answer:
(1) CH4 – Methane
(2) Ethane – C2H6
(3) Alkene – CH2 = CH2
(4) Alkyne – CnH2n – 2.
![]()
Question 2.
| Column I | Column II |
| (1) Aromatic hydrocarbon | (a) Propyne |
| (2) Alkane | (b) Benzene |
| (3) Alkyne | (c) Saturated hydrocarbon |
| (4) Alkene | (d) CnH2n |
| (e) C n H2n – 1 OH |
Answer:
(1) Aromatic hydrocarbon – Benzene
(2) Alkane – Saturated hydrocarbon
(3) Alkyne – Propyne
(4) Alkene – CnH2n.
Question 3.
| Column I | Column II |
| (1) Cyclohexane | (a) CH3COOH |
| (2) Methanol | (b) CH3Cl |
| (3) Acetaldehyde | (c) CH2Cl2 |
| (4) Ethanoic acid | (d) CH3OH |
| (e) C6H12 | |
| (f) CH3CHO |
Answer:
(1) Cyclohexane – C6H12
(2) Methanol – CH3OH
(3) Acetaldehyde – CH3CHO
(4) Ethanoic acid – CH3COOH.
Question 4.
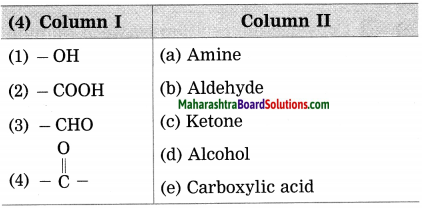
Answer:
(1) (-OH) – Alcohol
(2) (-COOH) – Carboxylic acid
(3) (-CHO) – Aldehyde
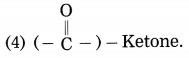
Question 5.
| Column I | Column II |
| (1) Ethyne | (a) C2H6 |
| (2) Ethene | (b) C2H2 |
| (3) Ethane | (c) C3H6 |
| (4) Propyne | (d) C2H4 |
| (e) C3H4 |
Answer:
(1) Ethyne – C2H2
(2) Ethene – C2H4
(3) Ethane – C2H6
(4) Propyne – C3H4.
![]()
Question 6.
| Column I | Column II |
| (1) Cellulose | (a) P.V.C. pipes, bags |
| (2) R.N.A | (b) Blankets |
| (3) Polyacrylonitrile | (c) Wood |
| (4) Polyvinyl chloride | (d) Chromosomes of plants |
Answer:
(1) Cellulose – Wood
(2) R.N.A. – Chromosomes of plants
(3) Polyacrylonitrile – Blankets
(4) Polyvinyl chloride – P.V.C. pipes, bags.
Consider the relation between Column I and II. Fill in Column IV to match Column III.
| Column I | Column II | Column III | Column IV |
| (1) Ethylene | Polyethylene | Tetrafluoroethylene | —————– |
| (2) Polypropylene | Propylene | Polystyrene | —————– |
| (3) Polysaccharide | Glucose | Proteins | —————– |
| (4) Rubber | Isoprene | D.N.A. | —————– |
| (5) Wood | Cellulose | Chromosomes of plants | —————– |
Answer:
(1) Teflon
(2) Styrene
(3) Alpha aminoacid
(4) Nucleotide
(5) R.N.A.
Define the following:
Question 1.
Define Alkane
Answer:
Alkane: In hydrocarbon, the four valencies of carbon atom are satisfied only by the single bonds, such compounds are called alkane.
Example: Methane (CH4), Ethane (C2H6)
Question 2.
Define Alkene.
Answer:
Alkene: The unsaturated hydrocarbons containing a carbon-carbon double bond are called alkenes.
Example : Ethene (CH2 = CH2)
Question 3.
Define Alkyne.
Answer:
Alkyne: The unsaturated hydrocarbons containing a carbon-carbon triple bond are called alkynes.
Example: Ethyne C2H2 (CH ≡ CH).
![]()
Question 4.
Define Addition reaction.
Answer:
Addition reaction: When a carbon compound combines with another compound to form a product that contains all the atoms in both the reactants; it is called an addition reaction.
![]()
Question 5.
Define Substitution reaction.
Answer:
Substitution reaction: The reaction in which the place of one type of atom/group in a reactant is taken by another atom/group of atoms, is called substitution reaction.

Question 6.
Define Esterification.
Answer:
Esterification: A carboxylic acid reacts with an alcohol in presence of an acid catalyst, an ester is formed. The reaction is known as esterification.
Question 7.
Define Saponification.
Answer:
Saponification: When an ester reacts with the alkali, i.e. sodium hydroxide, the corresponding alcohol and sodium salt of carboxylic acid are obtained. This reaction is called saponification reaction. It is used in the preparation of soap.
Ester + Sodium hydroxide → Sodium salt of carboxylic acid + Alcohol.
Question 8.
Define Polymerization.
Answer:
Polymerization: The reaction by which monomer molecules are converted into a polymer is called polymerization.
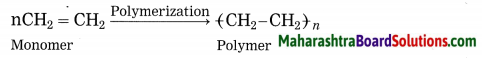
Name the following:
Question 1.
The higher homologue of hexane.
Answer:
Keptane.
Question 2.
The number of double bonds in benzene.
Answer:
Three.
Question 3.
The functional group in ether and halogen.
Answer:
Functional groups:
Ether: – O –
Halogen: – X (-Cl, -Br, -I).
![]()
Question 4.
Polymer of tetrafluoroethylene.
Answer:
Teflon.
Question 5.
The monomer of polysaccharide.
Answer:
Glucose.
Question 6.
The Polymer of nucleotide.
Answer:
D.N.A./R.N.A.
Question 7.
The Monomer of rubber.
Answer:
Isoprene.
Question 8.
Two oxidising compounds.
Answer:
Potassium permanganate, Potassium dichromate.
Question 9.
IUPAC name of sodium acetate.
Answer:
Sodium ethanoate.
Question 10.
The main component of natural gas.
Answer:
Methane.
Question 11.
Two isomers of butane.
Answer:
n-butane and i-butane.
Question 12.
A nomenclature system based on the structure of the compounds and it was accepted all over the world.
Answer:
International Union of Pure and Applied Chemistry (IUPAC).
Answer the following questions in one sentence each:
Question 1.
State the atomic number and electronic configuration of carbon.
Answer:
The atomic number of carbon is 6 and the electronic configuration of carbon is (2, 4).
Question 2.
State number of electrons in the outermost orbit of carbon and valency of carbon.
Answer:
Four electrons are present in the outermost orbit of carbon and the valency of carbon is 4.
Question 3.
What are hydrocarbons? Give one example.
Answer:
The compounds containing only carbon and hydrogen are called hydrocarbons. These compounds are known as organic compounds. E.g. Methane, Ethane.
Question 4.
What is the molecular formula and structural of methane?
Answer:
The molecular formula of methane is CH4.
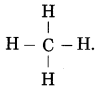
Question 5.
How many atoms of carbon and hydrogen are present in methane?
Answer:
The molecule of methane has one carbon atom and four hydrogen atoms.
Question 6.
State the general formula of alkane.
Answer:
The general formula of an alkane is CnH2n + 2.
![]()
Question 7.
Give two examples of alkanes.
Answer:
Methane (CH4) and ethane (C2H6) are alkanes.
Question 8.
Give two examples of alkenes.
Answer:
Ethene (CH2 = CH2) and propene (CH3 – CH = CH2) are alkenes.
Question 9.
Give two examples of alkynes.
Answer:
Ethyne (HC ≡ CH) and propyne (CH3 – C ≡ CH) are alkynes.
Question 10.
Write the name and molecular formula of a higher homologue of propane.
Answer:
Butane (C4H10) is a higher homologue of propane.
Question 11.
Write the structure and molecular formula of ethane.
Answer:
Structure of ethane:
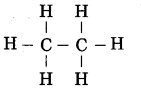
Molecular formula of ethane: C2H6
Question 12.
What is meant by catenation power?
Answer:
Carbon has a unique ability to form strong covalent bonds with other carbon atoms, this result in formation of big molecules. This property of carbon is called catenation power.
Question 13.
State the structural and molecular formula of benzene.
Answer:
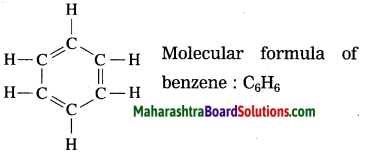
Question 14.
Which functional groups are present in aldehyde and ketone?
Answer:
The functional group -CHO is present in aldehyde and the functional group
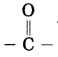
is present in ketone.
Question 15.
Which functional group is present in

Answer:
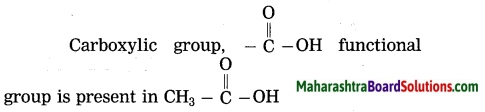
Question 16.
Compare: The proportions of carbon atoms in ethanol (C2H5OH) and naphthalene (C10H8).
Answer:
Ethanol contains two carbon atoms while naphthalene contains 10 carbon atoms. Ethanol is a saturated hydrocarbon and naphthalene is an unsaturated hydrocarbon.
Question 17.
What are the products of combustion of methane?
Answer:
Carbon dioxide (CO2) and water (H2O) are the products of combustion of methane.
Question 18.
Which gas is evolved when ethanol reacts with sodium?
Answer:
Hydrogen gas (H2) is evolved when ethanol reacts with sodium.
![]()
Question 19.
Compare: How is the transformation of ethanol into ethanoic acid on oxidation reaction?
Answer:
The transformation of ethanol into ethanoic acid is an oxidation process, in which ethanol accepts oxygen.
Question 20.
Complete the following:
![]()
Answer:

Question 21.
Which of the following hydrocarbons undergo addition reactions:
C2H6, C3H8, C2H4, C3H8?
Answer:
C2H4 (ethene) and C3H6 (propene) undergo addition reactions.
Question 22.
How many covalent bonds are there in a molecule of cyclohexane?
Answer:
A molecule of cyclohexane contains 18 covalent bonds.
Question 23.
Give the IUPAC name for CH3COOH.
Answer:
The IUPAC name for CH3COOH is ethanoic acid.
Question 24.
Write the IUPAC name of CH3COONa.
Answer:
IUPAC name of CH3COONa is sodium ethanoate.
Question 25.
What is meant by denatured alcohol?
Answer:
Ethanol is the important commercial solvent. To prevent the misuse of this solvent, it is mixed with the poisonous methanol. Such ethanol is called denatured spirit.
Question 26.
What is meant by glacial acetic acid ?
Answer:
The melting point of pure acetic acid is 17 °C. Therefore, during winter in old countries acetic acid freezes at room temperature itself and looks like ice. Therefore it is named glacial acetic acid.
Question 26.
Which useful components of hydro¬carbon are obtained by fractional distillation of crude oil?
Answer:
Various useful components of hydrocarbon such as CNG, LPG, petrol (gasoline), rockel, diesel, engine oil, lubricant, etc. are obtained by separation of crude oil using fractional distillation.
![]()
Question 28.
Which functional groups are present in ester and amine?
Answer:
Ester: -COO-
Amine: -NH2–
Question 29.
Give two examples of natural macromolecules.
Answer:
Examples: Polysaccharide, protein and nucleic acid.
Question 30.
Write the structure of polystyrene and give its uses.
Answer:
Structure:
Polystyrene is used to make thermocoal articles.
Question 31.
Write the name and the structure of monomer of polyacrylonitrile.
Answer:
The name and structure of monomer: Acrylonitrile CH2 = CH – CN
Question 32.
Write the name and the structure of monomer of teflon and its uses.
Answer:
The name and structure of monomer: Tetrafluro ethylene CF2 = CF2
Teflon is used to make nonstick cookware.
Question 33.
What is meant by copolymers?
Answer:
The polymers formed from two or more monomers are called copolymers.
Examples: Poly ethylene terephthalate.
Answer the following questions:
Question 1.
How is hydrogen molecule formed?
Answer:
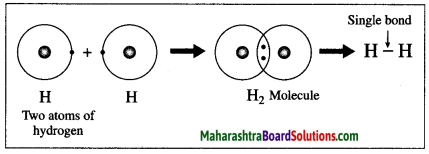
The atomic number of hydrogen is 1, its atom contains 1 electron in K shell. It requires one more electron to complete the K shell and attain the configuration of helium (He). To meet this requirement two hydrogen atoms share their electrons with each other to form H2 molecule. One covalent bond, i.e. a single bond is formed between two hydrogen atoms by sharing of two electrons.
Question 2.
Describe the formation of oxygen molecule (O2).
Answer:
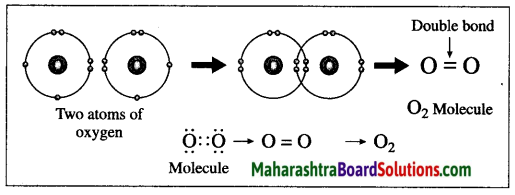
(1) The atomic number of oxygen is 8. The electronic configuration of oxygen is (2, 6). Oxygen has 6 electrons in the outermost shell.
(2) It requires 2 electrons to complete the L shell and attain the configuration of neon (Ne).
(3) Each oxygen atom shares its valence electron with the valence electron of another oxygen atom to give two shared pairs of electrons which results in the formation of oxygen molecule.
(4) Thus, two electron pairs are shared between two oxygen atoms, forming double covalent bond (=).
![]()
Question 3.
Describe the formation of nitrogen molecule.
Answer:
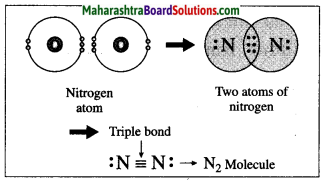
(1) The atomic number of nitrogen is 7. The electronic configuration of nitrogen is (2, 5). Nitrogen has 5 electrons in the outermost shell.
(2) It requires three more electrons to complete the L shell and attain the configuration of neon (Ne).
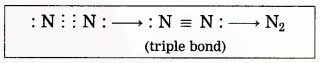
(3) Two nitrogen atoms come close together and share three pairs of electrons with each other, resulting in the formation of a triple bond.
(4) Thus, two nitrogen atoms are bound with a triple bond (=) to form a nitrogen molecule.
Question 4.
How is the methane molecule formed?
Answer:
(1) The electronic configuration of carbon is (2, 4). Carbon has four electrons in the outermost shell, hence it is tetravalent.
(2) The electronic configuration of hydrogen is 1, hence it is monovalent.
(3) Carbon needs four electrons to complete the L shell and attain the configuration of neon (Ne).
(4) Four atoms of hydrogen share 1 electron each with 4 electrons of carbon.
(5) A single covalent bond is formed by sharing of two electrons.
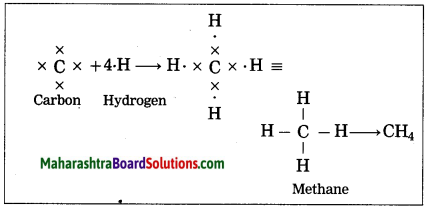
Thus, the methane molecule contains four single bonds between the carbon and hydrogen atoms.
Question 5.
State the various compounds and its formulae formed by a single atom of carbon with monovalent hydrogen and chlorine.
Answer:
| Compounds | Names |
| CH4 | Methane |
| CH3Cl | Methyl chloride |
| CH2Cl2 | Methylene dichloride |
| CHCl3 | Methylene trichloride |
| CCl4 | Carbon tetrachloride |
![]()
Question 6.
Observe the straight chain hydrocarbons given below and answer the following questions:
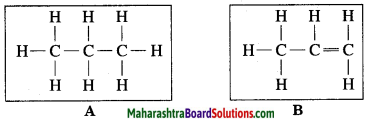
(i) Which of the straight chain compounds from A and B is saturated and unsaturated straight chains?
(ii) Name these straight chains.
(iii) Write their chemical formulae and number of -CH2 units. (Practice Activity Sheet – 2)
Answer:
(i) A is a saturated hydrocarbon, B is an unsaturated hydrocarbon.
(ii) A = Propane, B = Propene
(iii) The chemical formula of A = C3H8 and number of -CH2 units are 3.
The chemical formula of B = C3H6 and number of -CH2 unit is 1.
Question 7.
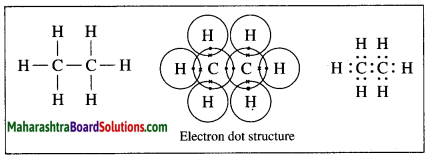
Draw electron-dot and line structure of an ethane molecule.
Answer:
The molecular formula of ethane is C2H6.
Question 8.
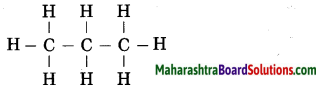
The molecular formula of propane is C3H8. From this draw its structural formula. (Practice Activity Sheet – 3)
Answer:
The structural formula of propane:
Question 9.
Draw the structure and carbon skeleton for cyclohexane.
Answer:
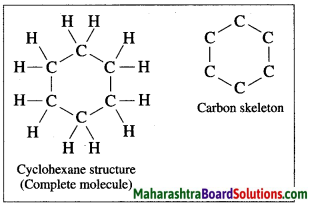
Question 10.
Classify into saturated and unsaturated hydrocarbons: (1) Methane (2) Ethene (3) Ethane (4) Ethyne (5) Propene (6) Propyne (7) Butane (8) Cyclohexene (9) Cyclopentane (10) Heptane.
Answer:
(i) Saturated hydrocarbons: (1) Methane (2) Ethane (3) Butane (4) Cyclopentane (5) Heptane.
(ii) Unsaturated hydrocarbons: (1) Ethene (2) Ethyne (3) Propene (4) Propyne (5) Cyclohexene.
Question 11.
Classify into alkanes, alkenes and alkynes: (1) Ethane (2) Ethene (3) Methane (4) Propene (5) Ethyne (6) Propyne (7) Butane (8) Pentane.
Answer:
Alkanes: (1) Ethane (2) Methane (3) Butane. (4) Pentane
Alkenes: (1) Ethene (2) Propene
Alkynes: (1) Ethyne (2) Propyne
![]()
Question 12.
Classify into straight chain carbon compounds, branched chain carbon compounds and ring carbon compounds:
(1) Propene (2) Butane (3) Iso-butane (4) Cyclopentane (5) Benzene (6) Isobutylene.
Answer:
Straight chain carbon compounds:
- Propene
- Butane.
Branched chain carbon compounds:
- Iso-butane
- Isobutylene.
Ring carbon compounds:
- Cyclopentane
- Benzene.
Question 13.
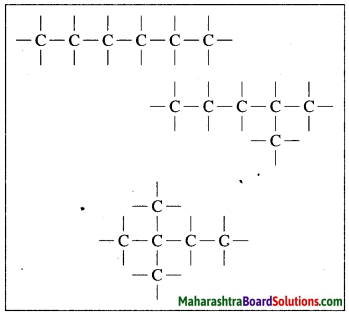
Draw chain and ring structures of organic compound having six carbon atoms in it.
Answer:
Chain structures of an organic compound having six carbon atoms:
Ring structures of an organic compound having six carbon atoms:
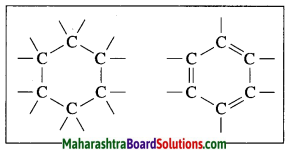
Question 14.
Explain the structure of benzene.
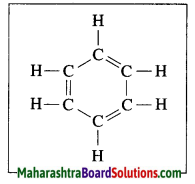
Answer:
The molecular formula of benzene is C6H6. It is a cyclic unsaturated hydrocarbon. Benzene ring is made of six carbon atoms. In benzene, each carbon atom is linked to two other carbon atoms, on one side by a single bond and on the other side by a double bond, i.e. three alternate single bonds and double bonds in the six membered ring structure of benzene. The compound having this characteristic unit in their structure are called aromatic compounds.
Question 15.
Draw the structures of isomers of pentane (C5H12).
Answer:
![]()
Question 16.
Recognize the carbon chain type for each of the following:
(Practice Activity Sheet – 1)
Answer:
In the reaction of acetic acid with sodium carbonate, carbon dioxide gas is evolved which turns lime water milky resulting in the formation of insoluble calcium carbonate.
Question 17.
What is meant by functional group? Give examples.
(OR)
Explain the term functional group with example.
Answer:
The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of length and nature of the carbon chain in that compound. Therefore these hetero atoms or groups of atoms containing hetero atoms are called the functional groups.
Example: Methyl alcohol, acetic acid.
In methane (CH4), when one hydrogen atom is replaced by an -OH group, methyl alcohol (CH3OH), is formed. The -OH is known as the alcoholic functional group.
Similarly, from methane (CH4) when one hydrogen atom is replaced by -COOH group, acetic acid (CH3COOH) is formed. The -COOH group is known as the carboxylic acid functional group.
Question 18.
Define functional group and complete the following table:
| Functional group | Compound | Formula |
| ——————– | Ethyl alcohol | ——————– |
| ——————– | Acetaldehyde | ——————– |
Answer:
The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of length and nature of the carbon chain in that compound. Therefore these hetero atoms or groups of atoms containing hetero atoms are called the
functional groups.
| Functional group | Compound | Formula |
| -OH | Ethyl alcohol | C2H5OH |
| -CHO | Acetaldehyde | CH3CHO |
![]()
Question 19.
What is meant by homologous series?
Answer:
The length of the carbon chains in carbon compounds is different their chemical properties are very much similar due to the presence of the same functional group in them. The series of compounds formed by joining the same functional group in place of a particular hydrogen atom on the chains having sequentially increasing length is called homologous series. Two adjacent members of the series differ by only one -CH2– (methylene) unit and their mass differ by 14 units.
The homologous series of straight chain alkanes can be represented by the general formula CnH2n + 2. The members of this series are as follows:
| Methane – CH4 Ethane – C2H6 |
– These differ by – CH2 units |
| Ethane – C2H6 Propane – C3H8 |
– These differ by – CH2 units |
| Butane – C4H10 Pentane – C5H12 |
– These differ by – CH2 units |
Question 20.
State the four characteristics of homologous series.
Answer:
Characteristics of Homologous series:
(1) In homologous series while going in an increasing order of the length of carbon chain
(a) one methylene unit ( -CH2– ) gets added
(b) molecular mass increases by 14 u (c) number of carbon atoms increases by one.
(2) Chemical properties of members of a homologous series show similarity due to the presence of the same functional group in them.
(3) Each member of the homologous series can be represented by the same general molecular formula.
(4) While going in an increasing order of the length there is gradation in the physical properties i.e. the boiling and melting points.
Question 21.
Write names of first four homologous series of alcohols: (March 2019)
Answer:
First four homologous series of alcohols are
- Methanol CH3 – OH
- Ethanol C2H5 – OH
- Propanol C3H7 – OH
- Butanol C4H9 – OH
![]()
Question 22.
Describe the IUPAC rules of naming organic compounds.
Answer:
IUPAC nomenclature system: International Union for Pure and Applied Chemistry (IUPAC) put forth a nomenclature system based on the structure of the compounds and it was accepted all over the world. There are three units in the IUPAC name of any carbon compound: parent, suffix and prefix. These are arranged in the name as follows:
Prefix-parent-suffix:
An IUPAC name is given to a compound on the basis of the name of its parent alkane. The name of the compound is constructed by attaching appropriate suffix and prefix to the name of the parent-alkane. The steps in the IUPAC nomenclature of straight chain compounds are as follows:
Step 1: Draw the structural formula of the straight chain compound and count the number of carbon atoms in it. The alkane with the same number of carbon atoms is the parent alkane of the concerned compound. Write the name of this alkane.
In case the carbon chain of concerned compound contains a double bond, change the ending of the parent name from ‘ane’ to ‘ene’. If the carbon chain in the concerned compound contains a triple bond, change the ending of the parent name from ‘ane’ to ‘yne’.
|
Sr. No. |
Structural formula | Straight chain |
Parent name |
| 1. | CH3 – CH2 – CH3 | C – C – C | propane |
| 2. | CH3 – CH2 – OH | C – C | ethane |
| 3. | CH3 – CH2 – COOH | C – C – C | propane |
| 4. | CH3 – CH2 – CH2 – CHO | C – C – C – C | butane |
| 5. | CH3 – CH = CH2 | C – C = C | propene |
| 6. | CH3 – C ≡ CH | C – C ≡ C | propyne |
Step 2: If the structural formula contains a functional group, replace the last letter ‘e’ from the parent name by the condensed name of the functional group as the suffix. (Exception: The condensed name of the functional group ‘halogen’ is always attached as the prefix.)
Step 3: Number the carbon atoms in the carbon chain from one end to the other. Assign the number T to carbon in the functional group -CHO or -COOH, if present. Otherwise, the chain can be numbered in two directions. Accept that numbering which gives smaller number to the carbon carrying the functional group. In the final name, a digit (number) and a character (letter) should be separated by a small horizontal line.
Question 23.
Write the IUPAC names of the following structural formulae.
a. CH3 – CH2 – CH = CH2
Answer:
Number of carbon atoms in the longest chain: 4
Parent alkane: Butene
Functional group: double bond
Assign the number:
![]()
In the longest chain, the numbering of carbon atom starts from the carbon atom nearest to the double bond and the other c-atoms are numbered accordingly.
Parent suffix: But-1-ene
IUPAC name: But-1-ene
b. CH3 – C ≡ C – H
Answer:
Number of carbon atoms in the longest chain: 3
Parent alkane: Propyne
Functional group: triple bond
Parent suffix: Propyne
IUPAC name: Propyne
c.
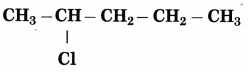
Answer:
The number of carbon atoms in the longest chain: 5
Parent alkane: Pentane
Prefix functional group: Chloro
Assign the number: 2
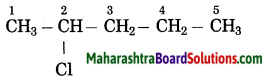
The carbon atom to which the -Cl atom is attached is numbered as C2 and the other C atoms are numbered accordingly.
Prefix parent: 2-Chloropentane
IUPAC name: 2-Chloropentane
![]()
d. CH3 – CH2 – CH2 – Br
Answer:
The number of carbon atoms in the longest chain: 3
Parent alkane : Propane Prefix functional group: Bromo
Assign the number:
![]()
The carbon atom to which the -Br atom is attached is numbered as C1 and the other C atoms are numbered accordingly.
Prefix parent: 1-Bromopropane
IUPAC name: 1-Bromopropane
e.
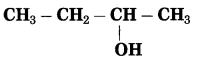
Answer:
The number of carbon atoms in the longest chain: 4
Parent alkane: Butane Functional group: -OH (ol)
Assign the number:
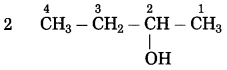
The carbon atom to which the -OH group is attached is numbered as C2.
If the carbon chain of the compound contains a -OH group, then change the ending ‘e’ of the parent name, i.e. ,‘e’ of butane is replaced by ‘ol’ (ol for alcohol).
Parent suffix: Butan-2-ol
IUPAC name: Butan-2-ol
f.
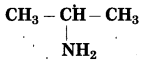
The number of carbon atoms: 3
Parent alkane: Propane
Functional group: -NH2 (amine)
Assign the number:
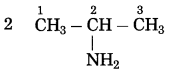
If the carbon chain of the compound contains a -NH2 group then change the ending of the parent name, i.e. ‘e’ of propane is replaced by ‘amine’.
Parent suffix: 2-Propanamine
IUPAC name: 2-Propanamine
g. HCOOH
Answer:
The number of carbon atoms: 1
Parent alkane: Methane
Functional group: -COOH (-oic cid)
If the carbon chain of the compound contains a -COOH group, then change the ending of the parent name, i.e. ‘e’ of methane is replaced by ‘-oic acid’.
Parent suffix: Methanoic acid
IUPAC name: Methanoic acid
h. CH3 – CH2 – CH2 – CHO
Answer:
The number of carbon atoms in the longest chain: 4
Parent alkane : Butane Functional group: -CHO (al)
Assign the number: 1
Assign the number ‘1’ to carbon in the functional group
![]()
If the carbon chain of the compound contains a -CHO group then change the ending of the parent name, i.e. ‘e’ of the butane is replaced by ‘al’.
Parent suffix: Butanal
IUPAC name: Butanal
i.
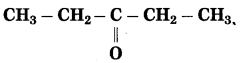
Answer:
The number of carbon atoms in the longest chain: 5
Parent alkane: Pentane
Functional group:
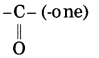
Assign the numbering:
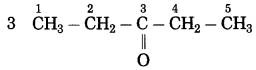
In the longest chain, the numbering of carbon atom starts from the carbon nearest to the functional group (both the numbering equivalent).
If the carbon chain of compound contains a
group, then change the ending of the parent name i.e. ‘e’ of pentane is replaced by ‘one’.
Parent suffix: Pentan-3-one
IUPAC name: Pentan-3-one.
![]()
Question 24.
What happens when methane is burnt in air? Write the balanced chemical equation for the same.
Answer:
When methane burns in air, carbon dioxide and water are formed. The reaction is exothermic with release of large amount of heat and light.
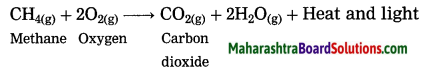
Question 25.
What happens when ethanol is burnt in air?
Answer:
When ethanol is burnt in air, it burns with a clean blue flame, carbon dioxide and water are formed. In this reaction, release of large amount of heat and light takes place.
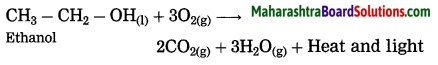
Question 26.
What happens when ethanol is treated with alkaline potassium permanganate? Write the balanced chemical equation for the same.
Answer:
When ethanol is treated with alkaline potassium permanganate, ethanol gets oxidised by alkaline potassium permanganate to’ form ethanoic acid.

Question 27.
What happens when vegetable oil is hydrogenated? Write the balanced chemical equation.
Answer:
When vegetable oil (unsaturated compound) is hydrogenated in the presence of nickel catalyst, vanaspati ghee (saturated) compound is formed.
Question 28.
What happens when chlorine is treated with methane?
(OR)
Describe the action of chlorine on methane.
(OR)
Write a note on chlorination of methane.
Answer:
Methane reacts rapidly with chlorine in the presence of sunlight to form four products. In this reaction, chlorine atoms replace, one by one, all the hydrogen atoms in the methane.
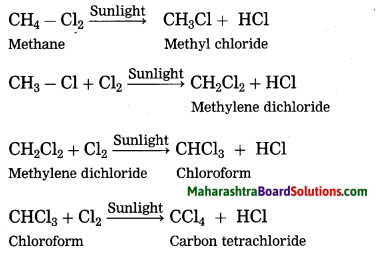
The reaction in which the place of one type of atom/group in a reactant is taken by another atom/group of atoms is called substitution reaction. Chlorination of methane is a substitution reaction.
Question 29.
What happens when ethanol is reacted with sodium?
Answer:
When ethanol is reacted with sodium at room temperature, sodium ethoxide is formed and hydrogen gas is liberated.

Question 30.
What happens when ethanol is heated at 170 °C with excess of conc. sulphuric acid?
Answer:
When ethanol is heated at 170 °C with excess of conc. sulphuric acid, one molecule of water is removed from its molecule to form ethene (unsaturated compound).

Question 31.
What happens when ethanoic acid is treated with sodium hydroxide? Write the balanced equation for the same.
Answer:
When ethanoic acid is treated with sodium hydroxide, neutralization takes place to form sodium acetate (sodium ethanoate) and water.
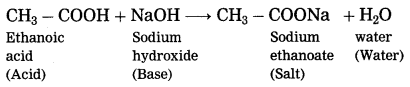
Question 32.
What happens when ethanoic acid is treated with sodium carbonate?
Answer:
When ethanoic acid is treated with sodium carbonate, sodium ethanoate, carbon dioxide and water is formed.
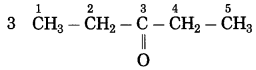
![]()
Question 33.
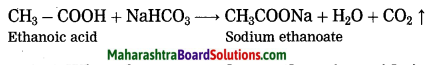
What happens when ethanoic acid is treated with sodium bicarbonate?
Answer:
When ethanoic acid is treated with sodium bicarbonate, sodium ethanoate, water and carbon dioxide is formed.
Question 34.
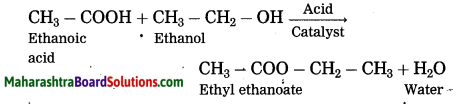
What happens when ethanoic acid is treated with ethanol? Write the balanced equation for the same.
Answer:
When ethanoic acid is treated with ethanol in the presence of an acid catalyst, an ester, i.e., ethyl ethanoate is formed.
Question 35.
What happens when ethylene gas is heated at high pressure and high temperature in the presence of suitable catalyst?
Ans. When ethylene gas is heated at high pressure and high temperature in the presence of suitable catalyst, it polymerizes to form polyethylene or polythene (plastic).
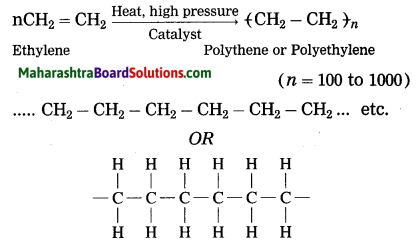
Question 36.
State the physical properties of ethyl alcohol ethanol.
Answer:
- Ethanol is a colourless liquid and it is soluble in water in all proportions and has pleasant odour.
- The boiling point of ethanol is 78 °C and the freezing point is -114 °C.
- It is combustible and burns with a blue flame.
- An aqueous solution of ethanol is neutral to litmus paper.
Question 37.
State the properties of ethanoic acid.
Answer:
- Ethanoic acid is a colourless liquid with boiling point 118 °C and melting point 17 °C. It has a pungent odour.
- Its aqueous solution is acidic and turns blue litmus red.
- A 5-8% aqueous solution of acetic acid is used as vinegar.
- It is a weak acid.
Write short notes:
Question 1.
Catenation power.
Answer:
(1) Carbon has a unique ability to form strong covalent bonds with other carbon atoms; this results in formation of big molecules. This property of carbon is called catenation power.
(2) Carbon shows catenation. Two or more carbon atoms can share their valence electrons and bond with each other. Thus, carbon chains can be straight or branched or closed chain ring structure forming large molecules. The covalent bond between two carbon atoms is strong and therefore stable. Carbon is bestowed with catenation power due to the strong and stable covalent bonds.
(3) Hence, carbon atoms can form an unlimited number of compounds.
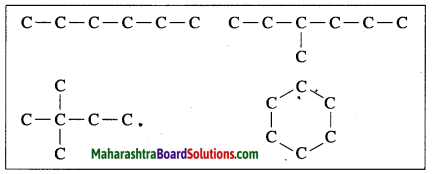
![]()
Question 2.
Characteristics of Carbon.
Answer:
(1) Carbon has a unique ability to form strong covalent bonds with other carbon atoms: this results in formation of big molecules. This property of carbon is called catenation power. The carbon compounds contain open chains or closed chains of carbon atoms. An open chain can be a straight chain or a branched chain. A closed chain is a ring structure. The covalent bond between two carbon atoms is strong and therefore stable. Carbon is bestowed with catenation power due to the strong and stable covalent bonds.
Question 3.
Functional group.
Answer:
(1) The compound acquire specific chemical properties due to these hetero atoms or the groups of atoms that contain hetero atoms, irrespective of the length and nature of the carbon chain in that compound. Therefore these hetero atoms or the groups of atoms containing hetero atoms are called functional groups.
All organic compounds are derivatives of hydrocarbons. The derivatives are formed by replacing one or more H-atom/atoms of hydrocarbon by some other hetero atom or groups of atoms containing hetero atoms. After replacement, a new compound is formed which has properties different from the parent hydrocarbon.
Examples: For methane, if one hydrogen atom is replaced by an – OH group, then a compound is methyl alcohol (CH3OH). The -OH group is known as the alcoholic functional group.
Functional group is organic compound:
1. Alcohol: – OH (hydroxy group)
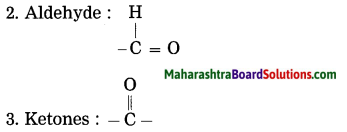
4. Carboxylic acid : -COOH
Question 4.
Homologous series.
Answer:
The length of the carbon chains in carbon compounds is different their chemical properties are very much similar due to the presence of the same functional group in them. The series of compounds formed by joining the same functional group in place of a particular hydrogen atom on the chains having sequentially increasing length is called homologous series. Two adjacent members of the series differ by only one -CH2– (methylene) unit and their mass differ by 14 units.
The homologous series of straight chain alkanes can be represented by the general formula CnH2n + 2
The members of this series are as follows:
| Methane – CH4 Ethane – C2H6 |
– These differ by – CH2 units |
| Ethane – C2H6 Propane – C3H8 |
– These differ by – CH2 units |
| Butane – C4H10 Pentane – C5H12 |
– These differ by – CH2 units |
Characteristics:
(1) In homologous series while going in an increasing order of the length of carbon chain
(a) one methylene unit (-CH2-) gets added
(b) molecular mass increases by 14 u
(c) number of carbon atoms increases by one.
(2) Chemical properties of members of a homologous series show similarity due to the presence of the same functional group in them.
(3) Each member of the homologous series can be represented by the same general molecular formula.
(4) while going in an increasing order of the length there is gradation in the physical properties i.e., the boiling and melting points.
![]()
Question 5.
Polymerization.
Answer:
(1) The reaction by which monomer molecules are converted into a polymer is called polymerization. A macromolecule formed by regular repetition of a small unit is called polymer. The small unit that repeats regularly to form a polymer is called monomer. The important method of polymerization is to make a polymer by joining alkene type of monomers.
(2) When ethylene gas is heated at high pressure and high temperature in the presence of suitable catalyst, it polymerizes to form polyethylene or polythene (plastic).
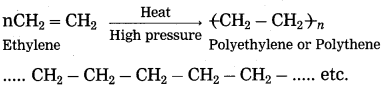
(3) The polymer polystyrene is used to make thermocoal articles. The polymer polyvinyl chloride is used to make P.V.C. pipes, doormats, etc. The polymer teflon is used to make nonstick cookware. The polymer polypropylene is used to make injection syringe, furniture, etc.
Give scientific reasons:
Question 1.
Carbon atoms are capable of forming an unlimited number of compounds.
Answer:
- Carbon has the property of catenation. Two or more carbon atoms can share some of their valence electrons to form (single, double and triple) bonds.
- The straight chains or branched chains or rings may have different shapes and sizes. This results in formation of many compounds. Hence, carbon atoms are capable of forming an unlimited number of compounds.
Question 2.
Ethylene is an unsaturated hydrocarbon.
Answer:
(1) Ethylene (CH2 = CH2) contains a double bond between carbon atoms.
(2) Thus, the valencies of the two carbon atoms are not fully satisfied by single covalent bonds. Hence, ethylene is an unsaturated hydrocarbon.
Question 3.
Naphthalene burns with a yellow flame.
Answer:
(1) Naphthalene is an unsaturated compound. In unsaturated hydrocarbon the proportion of carbon is larger than that of saturated hydrocarbon. As a result, some unburnt carbon particles are also formed during combustion of unsaturated compounds.
(2) In the flame. these unburnt hot carbon particles emit yellow light and therefore the flame appears yellow. Hence, naphthalene burns with a yellow flame.
Question 4.
The colour of iodine disappears in the reaction between vegetable oil and iodine.
Answer:
(1) Vegetable oils (unsaturated compound) contains a multiple bond as their functional group. They undergo addition reaction to form a saturated compound as the product.
(2) The addition reaction of vegetable oil with iodine takes place instantaneously at room temperature. The colour of iodine disappears in this reaction. This iodine test indicates the presence of a multiple bond in vegetable oil.
Question 5.
The hydrogenation of vegetable oil in the presence of nickel catalyst forms vanaspati ghee.
Answer:
(1) The molecules of vegetable oil contain long and unsaturated carbon chains. These unsaturated hydrocarbons contain a multiple bond as their functional group. They undergo addition reaction to form a saturated compound as the product.
(2) When vegetable oil (unsaturated compound) is hydrogenated in the presence of nickel catalyst, the addition reaction takes place, vanaspati ghee (saturated compound) is formed.
![]()
Distinguish between the following:
Question 1.
Saturated hydrocarbons and Unsaturated hydrocarbons.
Answer:
Saturated hydrocarbons:
- In saturated hydrocarbons, the carbon atoms are linked to each other only by single covalent bonds.
- They contain only a single bond.
- They are chemically less reactive.
- Substitution reaction is a characteristic property of these hydrocarbons.
- Their general formula is CnH2n + 2.
Unsaturated hydrocarbons:
- In unsaturated hydrocarbons, the valencies of carbon atoms are not fully satisfied by single covalent bonds.
- They contain carbon to carbon double or triple bonds.
- They are chemically more reactive.
- Addition reaction is a characteristic property of these hydrocarbons.
- Their general formula is CnH2n or CnH2n – 2
Question 2.
Open chain hydrocarbons and closed chain hydrocarbons.
Answer:
Open chain hydrocarbons:
- A hydrocarbon in which the chain of carbon atoms is not cyclic is called an open chain hydrocarbon.
- All aliphatic hydrocarbons contain open chains.
Closed chain hydrocarbons:
- A hydrocarbon in which the chain of carbon atoms is present in a cyclic form or ring form is called a closed chain hydrocarbon.
- All aromatic hydrocarbons contain closed chains.
Question 3.
Alkane and Alkene.
Answer:
Alkane
- Alkanes in which the carbon atoms are linked to each other only by single bonds.
- The general formula of an alkane is CnH2n + 2
- They are chemically less reactive.
Alkene:
- Alkenes in which carbon atoms are linked to each other by double bonds.
- The general formula of an alkene is CnH2n.
- They are chemically more reactive.
![]()
Project:
Question 1.
Prepare a list of carbon compounds which occur in nature and discuss their uses in daily life.
10th Std Science Part 1 Questions And Answers:
- Gravitation Class 10 Questions And Answers
- Periodic Classification of Element Class 10 Questions And Answers
- Chemical reactions and equations Class 10 Questions And Answers
- Effects of electric current Class 10 Questions And Answers
- Heat Class 10 Questions And Answers
- Refraction of light Class 10 Questions And Answers
- Lenses Class 10 Questions And Answers
- Metallurgy Class 10 Questions And Answers
- Carbon Compounds Class 10 Questions And Answers
- Space Missions Class 10 Questions And Answers