Class 8 Hindi Chapter 5 Kheti Se Aai Tabdiliyan Question Answer Maharashtra Board
Balbharti Maharashtra State Board Class 8 Hindi Solutions Sulabhbharati Chapter 5 खेती से आई तब्दीलियाँ Notes, Textbook Exercise Important Questions and Answers.
Std 8 Hindi Chapter 5 Kheti Se Aai Tabdiliyan Question Answer Maharashtra Board
Hindi Sulabhbharti Class 8 Solutions Chapter 5 खेती से आई तब्दीलियाँ Textbook Questions and Answers
सूचना के अनुसार कृतियाँ करो
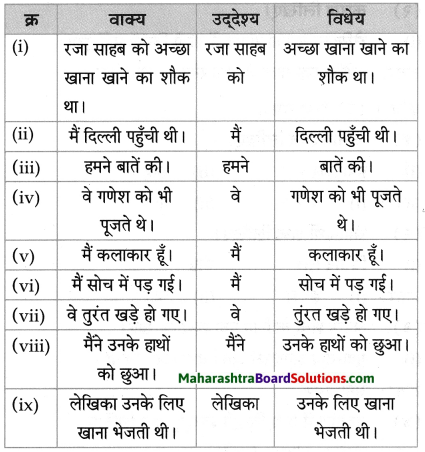
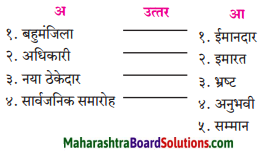
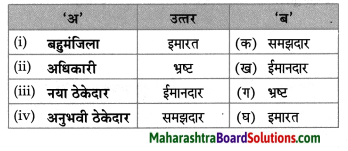
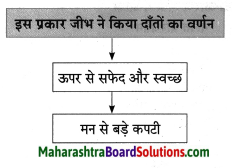
प्रवाह तालिका पूर्ण करो:
Question 1.

Answer:

![]()
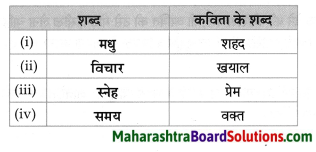
उचित जानकारी लिखो:
Question 1.

Answer:

कारण लिखिए।
Question 1.
मनुष्य को रोज शिकार खेलना पड़ता है।
Answer:
क्योंकि उसे स्वयं का गुजर बसर करना था।
Question 2.
अनाज सुरक्षित रखना प्रारंभ हुआ।
Answer:
जमीन में बहुत सारा अनाज पैदा होता था। इसे एकदम खाया नहीं जा सकता था।
Question 3.
मनुष्य को रोज शिकार खेलना पड़ता।
Answer:
स्वयं का पेट भरने के लिए उसे रोज शिकार खेलना पड़ता।
Question 4.
दुनिया में गरीब आदमी हैं।
Answer:
क्योंकि जो आज बिलकुल काम नहीं करता है; उसके पास बहुत सारा धन आ जाता है और जो दिन-रात पसीना बहाता है वह खाली हाथ रहता है। इस बुरे इंतजाम के कारण दुनिया में गरीब आदमी है।
Question 5.
लोग बैंक में रुपए रखते हैं।
Answer:
क्योंकि उनके पास अतिरिक्त रुपए होते हैं।
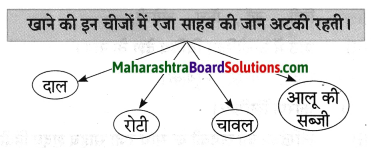
कृति पूर्ण करो:
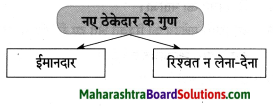
उस जमाने में उसे अमीर कहा जाता था जिसके पास ……….. थे
Question 1.

Answer:

![]()
भाषाबिंदु
निम्नलिखित वाक्यों में उचित विराम चिह्नों का प्रयोग कीजिए।
Question 1.
बड़े दुखी लग रहे हो क्या हुआ
Answer:
बड़े दुखी लग रहे हो, क्या हुआ?
Question 2.
अरे रामू के बापू घोड़े बेचकर सो रहे हो
Answer:
अरे, रामू के बापू, घोड़े बेचकर सो रहे हो?
Question 3.
तुम्हारे दाने कहाँ है
Answer:
तुम्हारे दाने कहाँ है?
उपयोजित लेखन
‘तबांकू सेवन के दुष्परिणाम’ विषय पर लगभग सौ शब्दों में निबंध लिखिए।
Answer:
तंबाकू सेवन सेहत के लिए हानिकारक होता है। तंबाकू में एक प्रकार का रासायनिक द्रव्य मिलाया जाता है जो शरीर नुकसान पहुँचाता है। तंबाकू में टार, निकोटीन व कार्बनमोनोक्साइड आदि पदार्थों को मिलाया जाता है। ये सारे पदार्थ मनुष्य के शरीर के लिए हानिकारक होते हैं। धीरे-धीरे उसका असर दिखाई देने लगता है। सबसे पहले वह मुँह को प्रभावित करता है और उसके बाद संपूर्ण शरीर को प्रभावित करना शुरू कर देता है। जो व्यक्ति तंबाकू का सेवन अधिक मात्रा में करते हैं उन्हें बाल झड़ने की समस्या से भी गुजरना पड़ता है।
तंबाकू का अति सेवन करने से मनुष्य को कैंसर जैसी बीमारियाँ होती हैं जिनका इलाज आज भी विज्ञान के लिए मुश्किल है। अत: बहुत सारे लोगों की तंबाकू को सेवन करने से मृत्यु हो रही है। कई लोग तंबाकू की लत के कारण यदि उन्हें समय पर खाने के लिए मिलता तो चिंतित एवं बेचैन हो जाते हैं। कई लोग तंबाकू का सेवन करना छोड़ना चाहते हैं और वे प्रयास भी करत हैं। लेकिन फिर भी वे अपनी इस आदत को छोड़ नहीं पाते हैं। तंबाकू का सेवन करने से व्यक्ति को हृदय संबंधी समस्याओं का भी सामना करना पड़ता हैं। तंबाकू का सेवन करने से मनुष्य को त्वचा संबंधी बीमारियाँ एवं नाखून झड़ने की समस्याओं का सामना करना पड़ता है। तंबाकू का सेवन मनुष्य के लिए जानलेवा और खतरनाक है। अतः मनुष्य को तंबाकू का सेवन कतई नहीं करना चाहिए।
शब्द समूह के लिए एक शब्द लिखिए।
Question 1.
जानवरों को चराने की जगह
Answer:
चरागाह
Question 2.
जिनके पास बहुत से अतिरिक्त रुपए हैं।
Answer:
- धनवान
- मालदार
स्वयं अध्ययन
पारंपरिक तथा आधुनिक कृषि प्रौद्योगिकी का तुलनात्मक चार्ट बनाओ।
![]()
Hindi Sulabhbharti Class 8 Solutions Chapter 5 खेती से आई तब्दीलियाँ Additional Important Questions and Answers
निम्नलिखित गद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए।
कृति क (१) आकलन कृति
समझकर लिखिए।
Question 1.

Answer:

गद्यांश के आधार पर वाक्य पूर्ण कीजिए।
Question 1.
तब काम बँटे हुए नहीं थे……
Answer:
जब शुरू में आदमी शिकार पर गुजर-बसर करता था।
Question 2.
जैसे-जैसे दिन गुजरने लगे……..
Answer:
वैसे-वैसे आदमियों ने नए नए पेशे सीखें और उनमें पक्के हो गए।
कृति क (२) आकलन कृति
उपर्युक्त गद्यांश से ऐसे दो प्रश्न तैयार कीजिए जिनके उत्तर निम्नलिखित शब्द हों –
Question 1.
औरत
Answer:
बच्चे और पालतू जानवरों की निगरानी कौन करता था?
Question 2.
काम
Answer:
कई हिस्सों में क्या बँट गए?
![]()
कृति क (३) शब्द संपदा
निम्नलिखित शब्द के तत्सम रूप लिखिए।
Question 1.
काम
Answer:
कार्य
निम्नलिखित शब्द मानक वर्तनी के अनुसार लिखिए।
Question 1.
नये-नये
Answer:
नए-नए
वचन बदलिए।
- बातें
- बेटी
Answer:
- बात
- बेटियाँ
नीचे दिए हुए शब्दों के पर्यायवाची शब्द लिखिए।
- हल
- मुश्किल
Answer:
- समाधान
- कठिन
Question 1.
गद्यांश में से लिंग शब्द की जोड़ी ढूँढ़कर लिखिए।
Answer:
आदमी – औरत
कृति क (४) स्वमत अभिव्यक्ति
Question 1.
आदिम अवस्था में मनुष्य किस प्रकार अपना गुजर-बसर करता था? इस बारे में जानकारी के आधार पर अपने विचार लिखिए।
Answer:
आदिम अवस्था में मानवीय सभ्यता का विकास नहीं हुआ था। उस अवस्था में मनुष्य के पास घर ही क्या साधारण-सी कुटिया भी नहीं थी। मानव जंगल में उपलब्ध कंदमूल खाया करता था और वहीं पर किसी एक पेड़ के नीचे सो जाया करता था। उसके पास शिकार के अतिरिक्त किसी भी प्रकार का काम नहीं होता था। अत: वह पूरा दिन शिकार की खोज में घूमा करता था। शिकार करने के शस्त्रों के अलावा मनुष्य के पास अन्य कोई साधन नहीं होते थे। उसके पास तन ढंकने के लिए कपड़े भी नहीं थे। वह पेड़ की पत्तियों से अपना शरीर ढंकते थे।
![]()
(ख) निम्नलिखित गद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए।
कृति ख (१) आकलन कृति
तुलना कीजिए।
Question 1.

Answer:

कारण लिखिए।
Question 1.
खेती के पहले लोग एक जगह नहीं ठहरते थे।
Answer:
क्योंकि शिकार हरेक जगह मिल जाता था। इसके अतिरिक्त गायों, बकरियों एवं दूसरे जानवरों की वजह से इधर-उधर घूमना पड़ता था।
कृति ख (२) आकलन कृति
एक शब्द में उत्तर लिखिए।
Question 1.
जानवरों को चरने के लिए इसकी जरूरत थी
Answer:
चरागाहों की .
Question 2.
खेती करना सीख जाने के बाद लोग इसके पास रहने लगे
Answer:
जमीन के
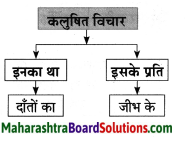
समझकर लिखिए।
Question 1.

Answer:

विलोम शब्द लिखिए।
- गाँव
- अच्छा
Answer:
- शहर
- बुरा
![]()
Question 1.
गद्यांश में से शब्द-युग्म ढूँढ़कर लिखिए।
Answer:
- इधर-उधर
- घूमते-फिरते
- जोत-बोकर
निम्नलिखित शब्दों में उचित प्रत्यय लगाइए।
- शिकार
- शहर
Answer:
- शिकारी
- शहरी
कृति ख (४) स्वमत अभिव्यक्ति
Question 1.
‘खेती करने के कारण समाज की निर्मिती हुई’ कथन के संदर्भ में अपने विचार लिखिए।
Answer:
खेती करने के पहले व्यक्ति इधर-उधर घूमता फिरता था। शिकार करके स्वयं का पेट पालता था। जैसे ही उसने खेती करना शुरू कर दिया वैसे ही वह जमीन के पास रहने लगा। परे साल भर वह खेती के काम में व्यस्त होने लगा। उसके आसपास रहने वालों की संख्या में वृद्धि होने लगी। वह एक-दूसरे के साथ संबंध प्रस्थापित करने लगा। आहिस्ता-आहिस्ता गाँव व कस्बे तैयार होने लगे। इससे समाज का निर्माण होने लगा।
कृति पूर्ण कीजिए।
Question 1.
खेती करने से आदमी को हुआ यह लाभ
Answer:
आदमी की जिंदगी ज्यादा आराम से कटने लगी।
समझकर लिखिए।
Question 1.

Answer:

![]()
सही विकल्प चुनकर वाक्य फिर से लिखिए।
Question 1.
अतिरिक्त खाने की वह …………. कर देता था। (जमा, खर्च, बाँट)
Answer:
जमा
Question 2.
आदमी को अपना ……….. भरने के लिए शिकार करना पड़ता था। (घर, पेट, बस्ता )
Answer:
पेट
कृति ग (३) शब्द संपदा
पर्यायवाची शब्द लिखिए।
- पैदा
- शिकार
- जमीन
- शिकारी
Answer:
- उपज
- आखेट
- भूमि
- आखेटक
Question 1.
गद्यांश में ऐसे दो शब्द बूडकर लिखिए जिनके वचन परिवर्तित नहीं होते।
Answer:
- पेट
- शिकार
विलोम शब्द लिखिए।
- बेकसूर x ………..
- ज्यादा x …….
Answer:
- कसूरवार
- कम
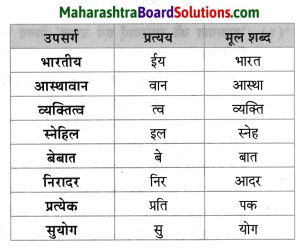
निम्नलिखित शब्दों से उपसर्ग को अलग कीजिए।
- अतिरिक्त
- सुरक्षित
Answer:
- अति
- सु
उचित प्रत्यय लगाइए।
- बात
- दिन
Answer:
- ऊनी : बातूनी
- इक : दैनिक
![]()
कृति ग (४) स्वमत अभिव्यक्ति
Question 1.
‘धन और अनाज को हमें भविष्य के लिए संग्रह रखना चाहिए।’ अपने विचार लिखिए।
Answer:
मनुष्य भले ही वर्तमान में जीवन व्यतीत करता है फिर भी उसे भविष्य के बारे में सोचना चाहिए। उसे भविष्य के लिए धन व अनाज जैसी चीजों का संग्रह करके रखना चाहिए। वर्तमान में हमारे पास यदि जरूरत से ज्यादा कुछ आता है तो हमें सब का सब खर्च नहीं करना चाहिए। हो सकता है कि भविष्य में हम पर कोई मुसीबत आ जाए और ऐसे में हमारे पास कुछ न होने से हमें यातनाओं का सामना करना पड़े। इसलिए व्यक्ति को दूर की सोच रखते हुए भविष्य के लिए धन या अनाज इकट्ठा करके रखना चाहिए।
निम्नलिखित गलत वाक्य सही करके फिर से लिखिए।
Question 1.
बैंक में बच्चे अनाज जमा करते हैं और चेक से निकाल भी सकते हैं।
Answer:
बैंक में लोग रूपए जमा करते हैं और चेक से निकाल भी सकते हैं।
Question 2.
जिनके पास अतिरिक्त रूपया होता है वही लोग अफसर होते हैं।
Answer:
जिनके पास अतिरिक्त रूपया होता है वही लोग मालदार होते हैं।
कृति घ (२) आकलन कृति
गद्यांश के आधार पर वाक्य पूर्ण कीजिए।
Question 1.
वे लोग गरीब कहलाते हैं
उत्तर
जिनके पास रुपए या अनाज नहीं होता है।
Question 2.
उसे खाली हाथ रहना पड़ता है
Answer:
जो दिन-रात पसीना बहाता है।
समझकर लिखिए।
Question 1.
यह है बुरा इंतजाम
Answer:
आज जो आदमी काम नहीं करता है उसके पास बहुत सारा धन आ जाता है और जो दिन-रात पसीना बहाता है वह खाली हाथ रहता है।
कारण लिखिए।
Question 1.
दुनिया में गरीब आदमी हैं।
Answer:
क्योंकि जो आज बिलकुल काम नहीं करता है। उसके पास बहुत सारा धन आ जाता है और जो दिन-रात पसीना बहाता है वह खाली हाथ रहता है। इस बुरे इंतजाम के कारण दुनिया में
गरीब आदमी है।
Question 2.
लोग बैंक में रुपए रखते हैं।
Answer:
क्योंकि उनके पास अतिरिक्त रुपए होते हैं।
कृति घ (३) शब्द संपदा
विलोम शब्द लिखिए।
- ज्यादा x ……..
- अमीर x ………
Answer:
- कम
- गरीब
समानार्थी शब्द लिखिए।
- गरीब
- पसीना
Answer:
- दीन
- स्वेद
निम्नलिखित अनेक शब्द के लिए एक शब्द लिखिए।
Question 1.
जिसके पास बहुत धन होता है।
Answer:
धनवान
Question 2.
जिनके पास बहुत से अतिरिक्त रुपए हैं।
Answer:
मालदार
Question 3.
गद्यांश में से शब्द-युग्म ढूँढ़कर लिखिए।
Answer:
- बहुत-सा
- आज-कल
लिंग बदलिए।
- पिता
- आदमी
Answer:
- माता
- औरत
![]()
Question 1.
‘आज अमीर अधिक अमीर होता जा रहा है और गरीब अधिक गरीब होता जा रहा है।’ पर अपने विचार लिखिए।
Answer:
आज हम नए दौर में जीवन जी रहे हैं। इस नए युग में समाज में ढेर सारी समस्याएं हैं। उनमें से एक अत्यंत गंभीर समस्या है – महँगाई। आज वस्तुओं की कीमतें आसमान को छू रही हैं। कर्मचारी एवं मजदूर जैसे सामान्य वर्ग के लोग पैसों की कमी होने के कारण अपने परिवार की जरूरतों को पूरा करने में असमर्थ सिद्ध हो रहे हैं। इस कारण वे रुपयों को भविष्य के लिए संग्रह नहीं कर पा रहे हैं।
इस कारण उनकी आर्थिक स्थिति में सुधार नहीं हो रहा है। समाज में रहने वाला अमीर वर्ग बिना कुछ काम किए अधिक से अधिक अमीर होता जा रहा है। इसका प्रमुख कारण यह है कि वे गरीबों को उनके काम की बहुत ही कम मजदूरी दे रहे हैं। यही कारण है कि अमीर अधिक अमीर होता जा रहा है और गरीब अधिक गरीब। गरीबों पर होनेवाले शोषण को रोक कर ही हम एक स्वस्थ समाज की स्थापना कर सकते हैं।
Hindi Sulabhbharati 8th Standard Digest Guide दूसरी इकाई
- धरती का आँगन महके Question Answer
- दो लघुकथाएँ Question Answer
- लकड़हारा और वन Question Answer
- सौहार्द -सौमनस्य Question Answer
- खेती से आई तब्दीलियाँ Question Answer
- अंधायुग Question Answer
- स्वराज्य मेरा जन्मसिद्ध अधिकार है Question Answer
- मेरा विद्रोह Question Answer
- नहीं कुछ इससे बढ़कर Question Answer