Std 12 Hindi Chapter 9 Chuninda Sher Question Answer Maharashtra Board
Balbharti Maharashtra State Board Hindi Yuvakbharati 12th Digest Chapter 9 चुनिंदा शेर Notes, Textbook Exercise Important Questions and Answers.
Hindi Yuvakbharati 12th Digest Chapter 9 चुनिंदा शेर Questions And Answers
12th Hindi Guide Chapter 9 चुनिंदा शेर Textbook Questions and Answers
कृति-स्वाध्याय एवं उत्तर
आकलन
प्रश्न 1.
(अ) लिखिए :
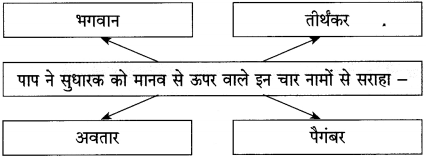
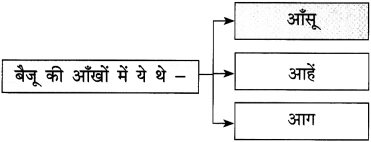
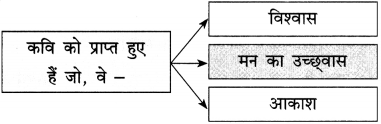
(a) परिंदों को यह शिकायत है –
उत्तर :
परिंदों को यह शिकायत है, हे मालिक कभी तो हमारी बात सुनो। ऐसा प्रतीत होता है कि जो दाना आपकी कृपा से हमें प्राप्त होता है, उसमें भी कीड़े लगे हैं।
![]()
(b) नदी के प्रति उत्तरदायित्व –
उत्तर :
नदी के प्रति उत्तरदायित्व – हमारी संस्कृति में नदी को माता के रूप में पूजा जाता है। नदी मानव सभ्यता के लिए जीवनदायिनी का काम करती है। इस नदी रूपी माता के लिए हमारा भी कुछ उत्तरदायित्व है। हमें नदी को स्वच्छ रखना चाहिए। कूड़ा-कचरा, रसायन नदी में नहीं डालने चाहिए।
(आ) परिणाम लिखिए :
(a) पानी सर से गुजर जाएगा तो – ………………………………………….
उत्तर :
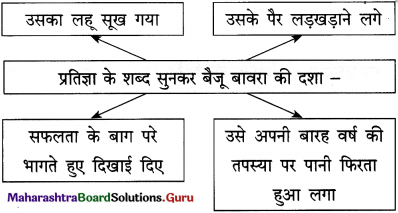
पानी सर से गुजर जाएगा तो – पानी सर से गुजर जाने का अर्थ है परिस्थिति का हाथों से निकल जाना। ऐसी स्थिति आने पर या तो व्यक्ति बिलकुल हताश हो जाता है या विद्रोही बनकर न करने योग्य कार्य भी कर गुजरता है।
(b) कवि जिंदगी के सवालों में खो गए – ………………………………………….
उत्तर :
कवि जिंदगी के सवालों में खो गए तब ऐसा हआ कि कवि के सवालों के जवाब उनके उजालों में खो गए।
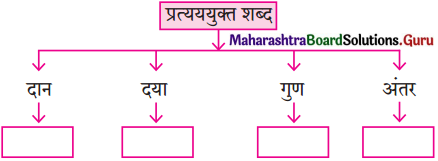
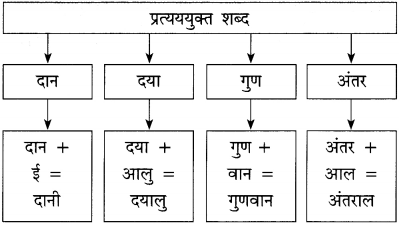
शब्द संपदा
प्रश्न 2.
पाठ में आए चार उर्दू शब्द और उनके हिंदी अर्थ :
(1) ………………… = …………………
(2) ………………… = …………………
(3) ………………… = …………………
(4) ………………… = …………………
उत्तर :
(1) खुशबू – सुगंध
(2) परिंदे – पक्षी
(3) ख्वाब – स्वप्न
(4) जिंदगी – जीवन।
![]()
अभिव्यक्ति
प्रश्न 3.
(अ) ‘आकाश के तारे तोड़ लाना’, इस मुहावरे को स्पष्ट कीजिए।
उत्तर :
आकाश के तारे तोड़ लाना मुहावरे का अर्थ है असंभव काम करना। जब कोई व्यक्ति किसी ऐसे कार्य की पूर्ति कर दे, जिसे कर पाना असंभव माना जा रहा हो तब उसके इस असंभव कार्य के लिए उपर्युक्त मुहावरे का प्रयोग किया जाता है। असीमित कठिनाइयों से भरा कोई काम, जिसे कर पाने में सभी असहज हों, वह कार्य विशेष कर पाना सभी को असंभव लगे, तब यह मुहावरा दोहराया जाता है। जैसे – तुम्हें क्या लगता है कि नलिन कुछ कर नहीं सकता। अरे… समय आने पर वह आकाश के तारे भी तोड़कर ला सकता है।
(आ) ‘क्रांति कभी भी अपने-आप नहीं आती; वह लाई जाती हैं, इस कथन पर अपने विचार लिखिए।
उत्तर :
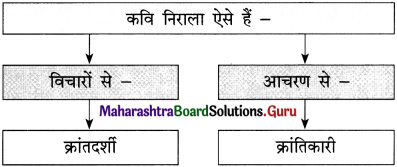
क्रांति अर्थात बदलाव लाना। बदलाव शासन व्यवस्था के प्रति हो सकता है या फिर किसी सामाजिक प्रथा के विरोध में। क्रांति कभी भी अपने-आप नहीं आती। क्रांति के लिए मानव को ही प्रयास करना पड़ता है। कोई व्यवस्था अथवा रूढ़ि भले ही जर्जर हो चुकी हो, समाज के विकास के लिए अहितकर बन रही हो।
अगर हम उसे बदलने के लिए क्रांतिकारी कदम नहीं उठाएँगे, तो हमारा समाज प्रगति नहीं कर पाएगा, कूपमंडूक बना रहेगा। इतिहास साक्षी है कि जब-जब मानव ने नए सिद्धांतों को, नई खोजों को अपनाया, समाज निरंतर विकास के मार्ग पर आगे बढ़ता रहा।
रसास्वादन
प्रश्न 4.
(अ) कवि की भावुकता और संवेदनशीलता को समझते हुए ‘चुनिंदा शेर’ का रसास्वादन कीजिए।
उत्तर :
कवि अपनी जिंदगी में आई परेशानियों से अप्रभावित हुए बिना उनका इस प्रकार सामना करते रहे कि वहीं से मानो उजाले फूट पड़े। सारी परेशानियाँ इस प्रकार समाप्त हो गईं मानो कभी थीं ही नहीं। हर सुबह हमारे लिए एक नया संदेश लेकर आती है। रात्रि के घोर अंधकार में जुगनू द्वारा फैलाए गए हल्के से प्रकाश में भी आशा की एक किरण छिपी होती है। कवि नित्य नए सपने देखता था, जागती आँखों के सपने।
वह नहीं जानता था कि उसके सपनों में, उसके विचारों में क्रांति का बीज छिपा है। उसके द्वारा आसमान पर लिखे गए सपने एक दिन क्रांति का रूप ले लेंगे। हँसी और आँसू मनुष्य के जीवन के दो अंग हैं। परंतु आज हर मनुष्य अपने जीवन की विसंगतियों से इस कदर त्रस्त है कि वह नहीं चाहता कि दूसरा कोई भी अपने आँसुओं से उसका कंधा भिगोए। अतः हमें अपने चेहरे पर एक मुखौटा लगाकर अपने आँसुओं को हँसी से छिपा लेना चाहिए।
![]()
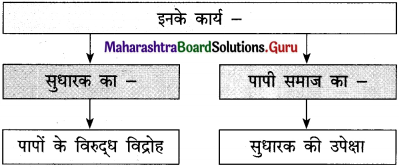
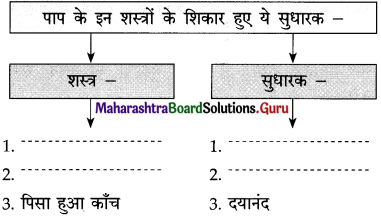
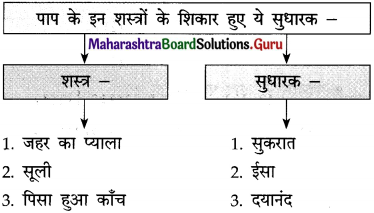
ईश्वर फकीरों, साधुओं और समाज की भलाई की इच्छा रखने वाले लोगों को ऐसी शक्ति प्रदान करता है कि उनके मुख से निकले आशीर्वाद सच होने लगते हैं। ऐसे लोगों की आँखें मानो करुणा और स्नेह बरसाती रहती हैं। हर मनुष्य की सहनशक्ति की एक सीमा होती है। प्रतिकूल परिस्थितियों, असफलताओं और अन्याय को सहन करने की शक्ति जिस दिन समाप्त हो जाएगी, उस व्यक्ति का विवेक उसका साथ छोड़ देगा।
वह दिन बस विद्रोह का दिन होगा। जीवन में निरंतर मिलती निराशाओं के कारण आँखों से आँसू इस प्रकार बहते रहते हैं मानो बाढ़ आ गई हो। कभी-कभी तो ऐसा प्रतीत होता है कि यह जीवन नहीं, बल्कि अषाढ़ का महीना है और निरंतर बादल बरस रहे हैं। एक मेहनतकश इन्सान जेठ मास की कड़कती हुई धूप में नंगे पाँव डामर की जलती सड़क पर चला जा रहा है। उसके पैरों की उँगलियाँ जल रही हैं।
साथ ही दिलोदिमाग में निराशा और हताशा की आँधियाँ चल रही हैं, बिजलियाँ घुमड़ रही हैं। मनुष्य की साँसें निश्चित हैं अर्थात प्रत्येक मनुष्य अपने जीवन में कितना आयुष्य पाएगा, कितनी साँसें ले पाएगा, यह पूर्वनिश्चित है। कवि को ऐसा महसूस होता है मानो उनकी साँसें उनकी अपनी नहीं हैं। अपनी साँसों पर उनका कोई अधिकार नहीं है। इस संसार में अनगिनत लोग ऐसे हैं, जिनमें से किसी का सिर खुला है, तो किसी के पैर चादर से बाहर हैं।
ये लोग अपनी आवश्यकताओं की पूर्ति भी नहीं कर पाते। हे ईश्वर ऐसा कुछ करो कि सभी लोगों को आवश्यकता की हर चीज मिले। सभी अपना भरण-पोषण उचित ढंग से कर सकें। कल भूख और बीमारी के कारण जिस मजदूर की साँसें बंद हो गई, जो इस निर्मोही दुनिया को छोड़कर चला गया, वह अनपढ़ था, निरक्षर था। परंतु उसके भी अनगिनत सपने थे। सपने देखने के लिए किसी भी प्रकार की साक्षरता की आवश्यकता नहीं होती। वह रोज अपनी इच्छाओं, आकांक्षाओं को मानो किताब में लिखता रहता था।
साहित्य संबंधी सामान्य ज्ञान
प्रश्न 5.
(अ) कैलाश सेंगर जी की प्रसिद्ध रचनाओं के नाम – ……………………………………
उत्तर :
- सूरज तुम्हारा है (गजल संग्रह)
- यहाँ आदमी नहीं, जूते भी चलते हैं
- सुबह होने का इंतजार (कहानी संग्रह)
- अभी रात बाकी है (अनूदित साहित्य)
(आ) गजल इस भाषा का लोकप्रिय काव्य प्रकार है – ……………………………………
उत्तर :
उर्दू
प्रश्न 6.
कोष्ठक में दी गई सूचना के अनुसार काल परिवर्तन करके वाक्य फिर से लिखिए :
(1) एक-एक क्षण आपको भेंट कर देता हूँ। (सामान्य भविष्यकाल)
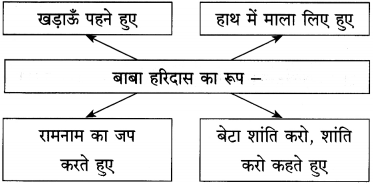
(2) बैजू का लहू सूख गया है। (सामान्य भूतकाल)
(3) मन बहुत दुखी हुआ था। (अपूर्ण भूतकाल)
(4) पढ़-लिखकर नौकरी करने लगा। (पूर्ण भूतकाल)
(5) यात्रा की तिथि भी आ गई। (सामान्य वर्तमानकाल)
(6) मैं पता लगाकर आता हूँ। (सामान्य भविष्यकाल)
(7) गर्ग साहब ने अपने वचन का पालन किया। (सामान्य भविष्यकाल)
(8) मौसी कुछ नहीं बोल रही थी। (अपूर्ण वर्तमानकाल)
(9) सुधारक आते हैं। (पूर्ण भूतकाल)
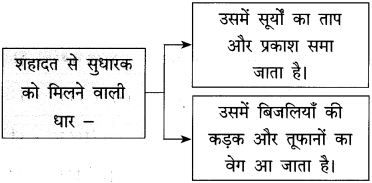
(10) प्रकाश उसमें समा जाता है। (सामान्य भूतकाल)
उत्तर :
(1) एक-एक क्षण आपको भेंट कर दूंगा।
(2) बैजू का लहू सूख गया।
(3) मन बहुत दुखी हो रहा था।
(4) पढ़-लिखकर नौकरी करने लगा था।
(5) यात्रा की तिथि भी आ जाती है।
(6) मैं पता लगाकर आऊँगा।
(7) गर्ग साहब अपने वचन का पालन करेंगे।
(8) मौसी कुछ नहीं बोल रही है।
(9) सुधारक आए थे।
(10) प्रकाश उसमें समा जाता था।
![]()
Hindi Yuvakbharati 12th Digest Chapter 9 चुनिंदा शेर Additional Important Questions and Answers
कृतिपत्रिका के प्रश्न 2 (अ) तथा प्रश्न 2 (आ) के लिए
पद्यांश क्र. 1
प्रश्न. निम्नलिखितपद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
कृति 1 : (आकलन)
प्रश्न 1.
निम्नलिखित शब्दों के लिए पद्यांश में प्रयुक्त शब्द ढूँढ़कर लिखिए :
(1) पक्षी – ………………………………………….
(2) सपना – ………………………………………….
(3) कला – ………………………………………….
(4) क्रांति – ………………………………………….
उत्तर :
(1) पक्षी – परिंदे
(2) सपना – ख्वाब
(3) कला – हुनर
(4) क्रांति – इन्कलाब
कृति 2 : (शब्द संपदा)
प्रश्न 2.
निम्नलिखित शब्दों के समानार्थी शब्द पद्यांश में से ढूँढ़कर लिखिए :
(1) निशा = कवि जिंदगी के सवालों में खो गए तब ऐसा हआ कि कवि के सवालों के जवाब उनके उजालों में खो गए।
(2) कुसुम = कवि जिंदगी के सवालों में खो गए तब ऐसा हआ कि कवि के सवालों के जवाब उनके उजालों में खो गए।
(3) प्रश्न = कवि जिंदगी के सवालों में खो गए तब ऐसा हआ कि कवि के सवालों के जवाब उनके उजालों में खो गए।
(4) स्वामी = कवि जिंदगी के सवालों में खो गए तब ऐसा हआ कि कवि के सवालों के जवाब उनके उजालों में खो गए।
उत्तर :
(1) निशा = रात
(2) कुसुम = फूल
(3) प्रश्न = सवाल
(4) स्वामी = मालिक।
पद्यांश क्र. 2
प्रश्न. निम्नलिखित पद्यांश पढ़कर दी गई सूचनाओं के अनुसार कृतियाँ कीजिए :
कृति 1 : (आकलन)
प्रश्न 1.
पद्यांश से दो ऐसे प्रश्न तैयार कीजिए, जिनके उत्तर निम्नलिखित हों :
(1) किताब
(2) अपनी साँस।
उत्तर :
(1) मजदूर रोज क्या लिखता था?
(2) कवि को क्या पराए धन-सी लगती है?
कृति 2 : (शब्द संपदा)
प्रश्न 1.
निम्नलिखित शब्दों का वचन बदलकर लिखिए :
(1) किताब – ……………………………….
(2) नदी – ……………………………….
(3) आँखों – ……………………………….
(4) उँगलियाँ – ……………………………….
उत्तर :
(1) किताब – किताबें
(2) नदी – नदियाँ
(3) आँखों – आँख
(4) उँगलियाँ – उँगली।
![]()
प्रश्न 2.
निम्नलिखित शब्दों के लिंग पहचानकर लिखिए :
(1) साँस – ……………………………….
(2) कंगन – ……………………………….
(3) सड़क – ……………………………….
(4) चादर – ……………………………….
उत्तर :
(1) साँस – स्त्रीलिंग
(2) कंगन – पुल्लिंग
(3) सड़क – स्त्रीलिंग
(4) चादर – स्त्रीलिंग।
रसास्वादन मुद्दों के आधार पर
कृतिपत्रिका के प्रश्न 2 (इ) के लिए
प्रश्न 1.
निम्नलिखित मुद्दों के आधार पर शेरों का रसास्वादन कीजिए :
उत्तर :
(1) रचना का शीर्षक : चुनिंदा शेर।
(2) रचनाकार : कैलाश सेंगर।
(3) कविता की केंद्रीय कल्पना : प्रस्तुत कविता में कवि की रचनाओं की प्रभावशीलता, परेशानियों से घबराए बिना उनका सामना करना, सुखद भविष्य के सपने देखना, उन्हें प्राप्त करने का प्रयास करने, आँसुओं को हँसी से छिपा लेना, त्याग और तपस्या के महत्त्व, समाज की भलाई की इच्छा, मनुष्य की सहन शक्ति की सीमा, विद्रोह, मेहनतकश इनसान के दिलोदिमाग में चलने वाली निराशा और हताशा की आँधियों का उल्लेख किया गया है। साथ ही इच्छा व्यक्त की गई है।
(4) रस-अलंकार :
(5) प्रतीक विधान : चट्टानी रातों को जुगनू से वह सँवारा करती है’ पंक्तियों में आशा की एक किरण के लिए जुगनू का प्रतीक के रूप में प्रयोग किया गया है।
(6) कल्पना : सामाजिक विषमता, अव्यवस्था तथा आम आदमियों की विवशताओं को अभिव्यक्त किया गया है।
(7) पसंद की पंक्तियाँ तथा प्रभाव : इसमें लाशें भी मिला करती हैं, तुम जरा देख-भाल तो लेते। इसको माँ कहके पूजनेवालों, इस नदी को खंगाल तो लेते। नदियों को माँ की तरह पूजनेवालों के लिए इन पंक्तियों में नदियोंको साफ-सुथरा रखने का आँख खोलनेवाला संदेश दिया गया है।
(8) कविता पसंद आने का कारण : इन पंक्तियों में कवि जलप्रदूषण रोकने की प्रेरणा दे रहे हैं। नदी मानव सभ्यता के लिए जीवन दायिनी का काम करती है। हमें नदी को स्वच्छ रखना चाहिए। कूड़ा-करकट, रसायन आदि नदी में नहीं डालने चाहिए।
अलंकार
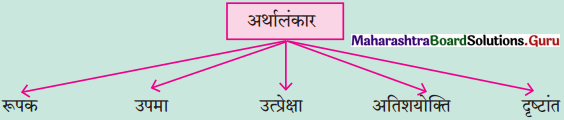
प्रश्न 1.
निम्नलिखित काव्य पंक्तियों में निहित अलंकार पहचानकर उसका नाम लिखिए :
(1) नहिं पराग, नहिं मधुर मधु, नहिं विकास इहिं काल।
अलि कलि ही सौं बिंध्यौ, आगे कौन हवाल।।
(2) सिर फट गया उसका, मानो अरुण रंग का घड़ा।
(3) वन शारदी चंदिका चादर ओढ़े।
उत्तर :
(1) अन्योक्ति अलंकार
(2) उत्प्रेक्षा अलंकार
(3) रूपक अलंकार।
![]()
रस
प्रश्न 1.
निम्नलिखित काव्य पंक्तियों में निहित रस पहचानकर है उसका नाम लिखिए :
(1) बतरस लालच लाल की मुरली धरी लुकाय।
सौंह करै, भौंहन हँसै, दै न कहि नटि जाय।।
(2) एक भरोसो, एक बल, एक आस विश्वास।
एक राम घनश्याम हित, चातक तुलसीदास।।
(3) आँखें निकाल उड़ जाते, क्षण भर उड़कर आ जाते।
शव जीभ खींचके कौवे, चुभला-चुभलाकर खाते।।
उत्तर :
(1) शृंगार रस
(2) भक्ति रस
(3) वीभत्स रस।
मुहावरे
प्रश्न 1.
निम्नलिखित मुहावरों के अर्थ लिखकर वाक्य में प्रयोग कीजिए :
(1) चोर की दाढ़ी में तिनका
अर्थ : अपराधी का भयभीत और सशंकित रहना।
वाक्य : दरोगा साहब को चोर की दाढ़ी में तिनका के है सिद्धांत पर अपराधियों को पकड़ने में समय नहीं लगता था।
(2) डकार तक न लेना
अर्थ : सब कुछ हजम कर लेना।
वाक्य : भ्रष्टाचार में लिप्त लोग करोड़ों रुपए खाकर बैठ जाते हैं और डकार तक नहीं लेते।
(3) पाँचों ऊँगलियाँ घी में होना
अर्थ : चहुँ ओर लाभ होना।
वाक्य : जब तक नरेश अपने नाना के साथ कोलकाता में धंधा करता था, तब तक उसकी पाँचों ऊँगलियाँ घी में होती थी।
(4) पोंगा होना
अर्थ : नासमझ होना।
वाक्य : भोलाराम की बात मत करो, वह तो पोंगा है पोंगा।
(5) बात का धनी
अर्थ : वचन का पक्का।
वाक्य : सेठ जेठामल गुस्सैल जरूर हैं, पर बात के धनी हैं।
(6) मूंछ उखाड़ना
अर्थ : घमंड चूर-चूर कर देना।
वाक्य : गोल्डन समारा अखाड़े के बाहर दारा सिंह को बढ़-चढ़कर चुनैतियाँ दे रहा था, पर अखाड़े में उतरा, तो दारा सिंह ने पटक-पटक कर उसकी मूंछ उखाड़ ली।
![]()
वाक्य शुद्धिकरण
प्रश्न 1.
निम्नलिखित वाक्य शुद्ध करके लिखिए :
(1) यहाँ तक की मिट्टी प्रदूषन से अछूती नहीं रही।
(2) निराला जी अपने युग की विशिष्ठ प्रतीभा हैं।
(3) चारों तरफ खुशिया जूमती थीं।
उत्तर :
(1) यहाँ तक कि मिट्टी भी प्रदूषण से अछूती नहीं रही।।
(2) निराला जी अपने युग की विशिष्ट प्रतिभा हैं।
(3) चारों तरफ खुशियाँ झूमती थीं।
चुनिंदा शेर Summary in Hindi
चुनिंदा शेर कवि का परिचय
चुनिंदा शेर कवि का नाम : कैलाश सेंगरय। (जन्म 16 फरवरी, 1954.)
चुनिंदा शेर प्रमुख कृतियाँ : सूरज तुम्हारा है (गजल संग्रह), यहाँ आदमी नहीं, जूते भी चलते हैं, सुबह होने का इंतजार (कहानी संग्रह), अभी रात बाकी है (अनूदित साहित्य) आदि।
चुनिंदा शेर विशेषता : कैलाश सेंगर जी की कविताएँ सहज-सरल भाषा में लिखी गई हैं, जिनमें आम आदमी की जिंदगी में व्याप्त वेदना, भावना आदि की अभिव्यक्ति है। गजल, गीत, कविता, कहानी, नाटक और पत्रकारिता के क्षेत्र में आपका योगदान उल्लेखनीय है। कथानक के तीखेपन और मौलिक प्रयोगों के कारण कैलाश सेंगर अत्यंत लोकप्रिय हैं। विधा उर्दू कविता का लोकप्रिय प्रकार गजल है। इस विधा की लोकप्रियता के फलस्वरूप हिंदी साहित्य में भी इसने अपनी जगह बना ली है और प्रेम की भावभूमि से हटकर यथार्थ की जमीन पर खड़ी है।
चुनिंदा शेर विषय प्रवेश : प्रस्तुत गजलों में सामाजिक विषमता, अव्यवस्था, आम आदमी की विवशताओं को विभिन्न चित्र शब्दों के माध्यम से अभिव्यक्त किया गया है।
चुनिंदा शेर कविता का सरल अर्थ
(1) गजलों से खुशबू …………………………………………. हुनर देता है।
कैलाश जी का यह मानना है कि कवि अपनी गजलों से, अपनी कविताओं से खुशबू फैलाने में सक्षम होता है। वह अपनी कृतियों से चट्टानों पर भी फूल खिला सकता है अर्थात असंभव कार्य को संभव करके दिखा सकता है, क्रांति ला सकता है।
परिंदे ईश्वर से शिकायत कर रहे हैं कि हे मालिक कभी तो हमारी बात भी सुनो। ऐसा प्रतीत होता है कि जो दाना आपकी कृपा से हमें प्राप्त होता है, उसमें भी कीड़े लगे हैं। अर्थात आपकी कृपा भी अब प्रदूषित हो गई है।

कवि जिंदगी में आई परेशानियों से अप्रभावित हुए बिना उनका इस प्रकार सामना करते रहे कि वहीं से मानो उजाले फूट पड़े। सारी परेशानियाँ इस प्रकार समाप्त हो गईं मानो कभी थी ही नहीं।
![]()
कवि कहते हैं कि ऐसा प्रतीत होता है कि हर सुबह हमारे लिए एक नया संदेश लेकर आती है। रात्रि के घोर अंधकार में जुगनू है द्वारा फैलाए गए हल्के से प्रकाश में भी आशा की एक किरण छिपी होती है।
कवि नित्य नए सपने देखता था, जागती आँखों के सपने। वह नहीं जानता था कि उसके सपनों में, उसके विचारों में क्रांति का बीज छिपा है। उसके द्वारा आसमान पर लिखे गए सपने एक दिन क्रांति का रूप ले लेंगे।
हँसी और आँसू मनुष्य के जीवन के दो अंग हैं। परंतु आज है हर मनुष्य अपने जीवन की विसंगतियों से इस कदर त्रस्त है कि वह नहीं चाहता कि दूसरा कोई भी अपने आँसुओं से उसका कंधा भिगोए। अतः अच्छा यही रहेगा कि अपने चेहरे पर एक मुखौटा लगाया जाए और अपने आँसुओं को हँसी से छिपा लिया जाए।
कवि त्याग और तपस्या के महत्त्व पर प्रकाश डालते हुए कहते हैं कि ईश्वर फकीरों, साधुओं और समाज की भलाई की इच्छा रखने वाले लोगों को ऐसी शक्ति प्रदान करता है कि उनके मुख से निकले आशीर्वाद सच होने लगते हैं। ऐसे लोगों की आँखें मानो करुणा और स्नेह बरसाती रहती हैं।
(2) इसमें लाशें भी मिला करती हैं …………………………………………. इक किताब लिखता था।
कवि कहते हैं कि हमारी संस्कृति में नदी को माता के रूप में पूजा जाता है। नदी मानव सभ्यता के लिए जीवन दायिनी का काम करती है। इस नदी रूपी माता के लिए हमारा भी कुछ उत्तरदायित्व है। इसमें लोग लाशें तक बहा देते हैं। हमें नदी को स्वच्छ रखना चाहिए। कूड़ा-कचरा, रसायन आदि नदी में नहीं डालने चाहिए।
कवि कहते हैं कि हर मनुष्य की सहन शक्ति की एक सीमा होती है। प्रतिकूल परिस्थितियों, असफलताओं और अन्याय को सहन करने की शक्ति जिस दिन समाप्त हो जाएगी, उस व्यक्ति का विवेक उसका साथ छोड़ देगा, वह दिन बस विद्रोह का दिन होगा।
कवि कहते हैं कि जीवन में निरंतर मिलती निराशाओं के कारण आँखों से आँसू इस प्रकार बहते रहते हैं मानो बाढ़ आ गई हो। कभी-कभी तो ऐसा प्रतीत होता है कि यह जीवन नहीं, बल्कि अषाढ़ का महीना है और निरंतर बादल बरस रहे हैं।
एक मेहनतकश इन्सान का वर्णन करते हुए कवि कहते हैं कि वह जेठ मास की कड़कती हुई धूप में नंगे पाँव डामर की जलती सड़क पर चला जा रहा है। उसके पैरों की उँगलियाँ जल रही हैं। साथ ही दिलोदिमाग में निराशा और हताशा की आँधियाँ चल रही हैं, बिजलियाँ घुमड़ रही हैं।
कवि कहते हैं कि मनुष्य की साँसें निश्चित हैं अर्थात प्रत्येक मनुष्य अपने जीवन में कितना आयुष्य पाएगा, कितनी साँसें ले पाएगा, यह पूर्वनिश्चित है। कवि को ऐसा महसूस होता है मानो उनकी साँसें उनकी अपनी नहीं हैं। अपनी साँसों की संख्या पर उनका कोई अधिकार नहीं है। ठीक उसी प्रकार जैसे किसी दूसरे की धन-संपत्ति पर हमारा अधिकार नहीं होता। या जैसे हम आवश्यकता पड़ने पर अपने कंगन या अन्य कोई आभूषण किसी महाजन के पास गिरवी रख देते हैं। उसी प्रकार हमारी साँसें भी हमारी अपनी नहीं है।
कवि सृष्टि को बनाने वाले जीवनदाता से कहता है कि इस संसार में अनगिनत लोग ऐसे हैं, जिनमें किसी का सिर खुला है, तो किसी के पैर चादर से बाहर हैं। ये लोग अपनी आवश्यकताओं की पूर्ति भी नहीं कर पाते। हे ईश्वर ऐसा कुछ करो कि सभी लोगों को आवश्यकता की हर चीज मिले। सभी अपना भरण-पोषण उचित ढंग से कर सकें।
कवि कहते हैं कि कल भूख और बीमारी के कारण जिस मजदूर की साँसें बंद हो गईं, जो इस निर्मोही दुनिया को छोड़कर चला गया, वह अनपढ़ था, निरक्षर था। परंतु उसके भी अनगिनत सपने थे। सपने देखने के लिए किसी भी प्रकार की साक्षरता की आवश्यकता नहीं होती। वह रोज अपनी इच्छाओं, आकांक्षाओं को मानो किताब में लिखता रहता था।
![]()
चुनिंदा शेर मुहावरे : अर्थ और वाक्य प्रयोग
(1) चट्टानों पर फूल खिलना।
अर्थ : कड़ी मेहनत से खुशहाली पाना।
वाक्य : हिमानी ऐसी दृढनिश्चयी है कि यदि वह ठान ले तो चट्टानों पर फूल खिला सकती है।
(2) सिर से पानी गुजर जाना।
अर्थ : कष्ट या संकट का पराकाष्ठा तक पहुँच जाना, संयम अथवा सहने की शक्ति समाप्त हो जाना।
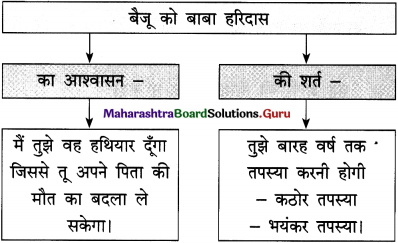
वाक्य : आए दिन सेठ की गालियाँ सुन-सुनकर गोपाल को लगा कि अब तो सिर से पानी गुजर गया और वह मालिक को टका-सा जवाब देकर नौकरी छोड़कर चला गया।
Hindi Yuvakbharati 12th Digest Maharashtra Board