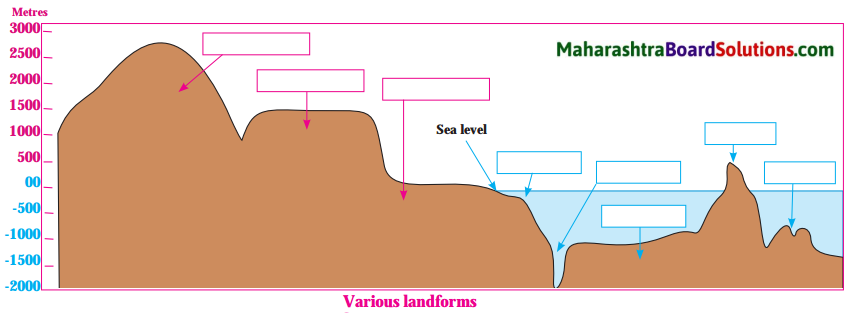
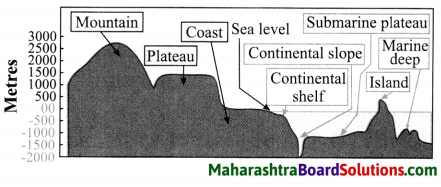
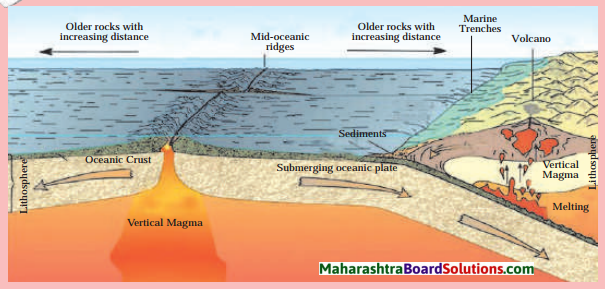
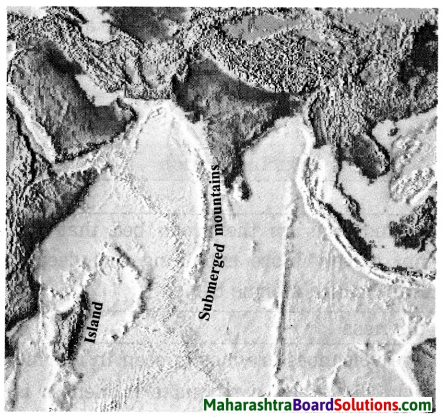
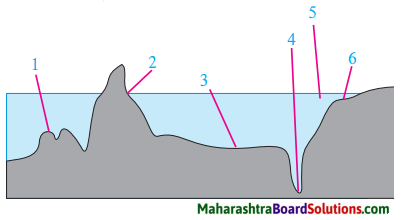
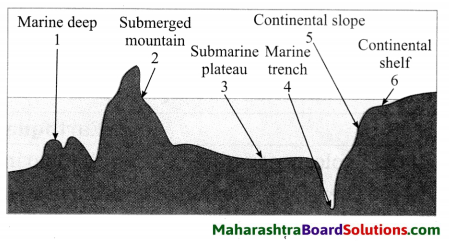
Balbharti Maharashtra State Board Class 8 Geography Solutions Chapter 5 Ocean Currents Notes, Textbook Exercise Important Questions and Answers.
Class 8 Geography Chapter 5 Ocean Currents Questions And Answers Maharashtra Board
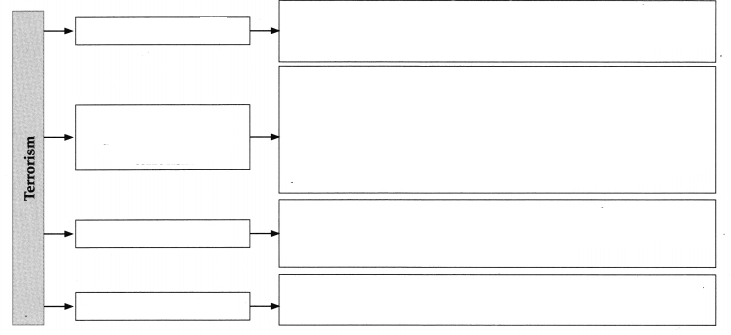
Ocean Currents Class 8 Questions And Answers Chapter 5 Maharashtra Board
Class 8 Geography Chapter 5 Ocean Currents Textbook Questions and Answers
1. Choose the correct option:
Question a.
In which ocean does the Labrador current flow?
(a) Pacific
(b) South Atlantic
(c) North Atlantic
(d) Indian
Answer:
(c) North Atlantic
![]()
Question b.
Which current out of the following flows in the Indian Ocean?
(a) East Australian current
(b) Peru current
(c) South Polar current
(d) Somali current
Answer:
(d) Somali current
Question c.
Which factor out of the following does not affect the region along the coast?
(a) Precipitation
(b) Temperature
(c) Land breeze
(d) Salinity
Answer:
(c) Land breeze
Question d.
Which of the following occurs in the area where the cold and warm currents meet?
(a) High temperature
(b) Snow
(c) Low temperature
(d) Thick fog
Answer:
(d) Thick fog
Question e.
Which of these following currents flow from the northern polar regiorTup to Antarctica?
(a) Warm ocean currents
(b) Surface ocean currents
(c) Cold ocean currents
(d) Deep ocean currents
Answer:
(d) Deep ocean currents
2. Examine the given statements and correct the wrong ones:
Question a.
Ocean currents give specific direction and velocity to the water.
Answer:
Correct.
![]()
Question b.
The deep ocean currents flow with high velocity.
Answer:
Correct.
Question c.
Generally, surface ocean currents are formed in the equatorial regions.
Answer:
Incorrect.
Correct statement: Generally, surface ocean currents are formed in the equatorial region as well as polar region.
Question d.
Ocean currents hold great importance for human life.
Answer:
Correct.
Question e.
The movement of icebergs is not dangerous for water transport.
Answer:
Incorrect.
Correct statement: The movement of icebergs is dangerous for water transport.
Question f.
Water becomes warm near Brazil due to ocean currents. On the other hand, it becomes cold near African coast.
Answer:
Incorrect.
Correct statement: Water becomes warm near Brazil due to ocean currents. Similarly, it also becomes warm near African coast.
3. Explain the effect of-
Question a.
Warm ocean currents on climate.
Answer:
- The amount of precipitation increases in the coastal region along with which the warm ocean currents flow.
- Similarly, the temperature increases in the coastal region along with which the warm ocean currents flow.
Question b.
Cold ocean currents on the movement of icebergs.
Answer:
- Due to cold ocean currents, icebergs are moved away from the polar regions.
- These icebergs come along the marine routes and prove hazardous to the ships.
![]()
Question c.
The shape of the coastline on ocean currents.
Answer:
- The extended parts of coastline acts as an obstacle for ocean currents.
- The extended parts of coastline alters the direction and velocity of ocean currents.
Question d.
Meeting of warm and cold ocean currents.
Answer:
- Dense fog is found in the meeting point of warm and cold ocean currents. Algae, plankton, etc. fish food grow on a large scale in these areas.
- Fish come in these areas on a large scale and breed. Therefore, extensive fishing grounds are found in the areas where warm and cold ocean currents meet.
Question e.
The transportation capacity of ocean currents.
Answer:
- The transportation capacity of ocean currents alters the amount of precipitation and temperature in the coastal regions along with which they flow.
- The transportation capacity of ocean currents leads to transfer of warm water to the bottom from the surface and cold water to the surface from the bottom.
Question f.
Deep ocean currents.
Answer:
- Deep ocean currents leads to transfer of warm water to the bottom from the surface and cold water to the surface from the bottom.
- Deep ocean currents thus leads to redistribution of sea water.
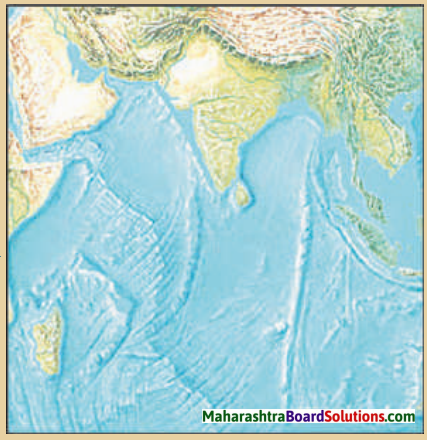
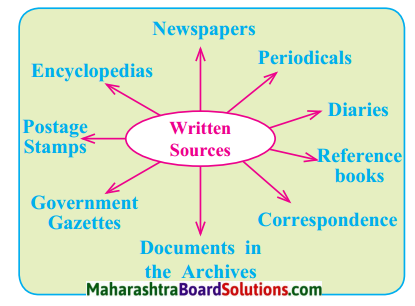
4. Look at the map of ocean currents and answer the following:
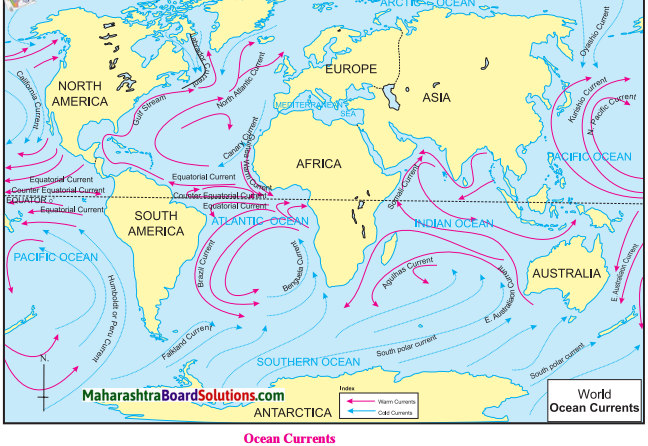
Question a.
How does the Humboldt current affect the climate of the South America?
Answer:
Humboldt current is a cold ocean current and so it decreases the amount of precipitation and temperature on the western coastal region of the South America.
![]()
Question b.
In which oceans are counter equatorial currents not observed and why?
Answer:
Counter equatorial currents are not observed in Arctic Ocean and Southern Ocean. These oceans are located near the polar regions. Therefore, the effects of trade winds is found to be less in these oceans Therefore, counter equatorial currents are not observed in these oceans.
Question c.
Which currents are absent in the northern part of the Indian Ocean and why?
Answer:
Cold currents are absent in the northern part of the Indian Ocean. The northern part of Indian Ocean is included in the temperate zone. So cold currents are absent in the northern part of the Indian Ocean.
Question d.
In which regions do the cold and warm ocean currents meet?
Answer:
The cold and warm ocean currents S meet in the following regions:
- North Atlantic Ocean (cold Labrador current and warm Gulf stream current)
- North Pacific Ocean (cold Oyashio current and warm ! Kurishio current)
- South Atlantic Ocean ! (cold Falkland current and warm Brazil current)
- South Pacific Ocean (cold South Polar current and warm East Australian current)
- Indian Ocean (cold South Polar i current and Agulhas Current)
5. Answer the following questions:
Question a.
What are the reasons responsible for the formation of deep ocean currents?
Answer:
- The temperature varies in various parts of ocean.
- Similarly, the density of water in various parts of ocean is also found to be different.
- The difference in the temperature and density of seawater leads to its circulation and the deep ocean currents are formed. This circulation is known as thermohaline circulation.
Thus, the difference in temperature and density of water in various parts of ocean are the reasons responsible for the formation of deep ocean currents.
![]()
Question b.
What is the reason behind the dynamics of the ocean water?
Answer:
Planetary winds is the reason behind the dynamics of the ocean water.
Question c.
How do winds give direction to the ocean currents?
Answer:
Winds give clockwise direction to the ocean currents in the northern hemisphere and anti-clockwise direction to the ocean currents in the southern hemisphere.
Question d.
Why do the ports in the eastern coast of Canada freeze in winter?
Answer:
- Labrador cold current flows along the eastern coast of Canada.
- Due to Labrador cold current, the temperature of sea water near the eastern coast of Canada decreases.
- Due to fall in temperature, the sea water along the eastern coast of Canada start freezing. As its effect, the ports in the eastern coast of Canada freeze in winter.
Activity:
Question a.
Look for more funny and interesting information related to ocean currents.
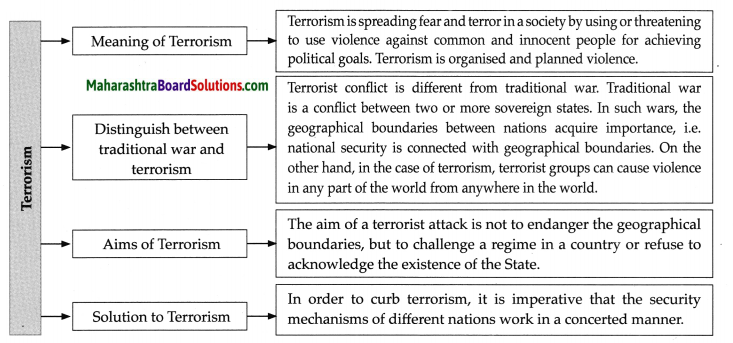
Class 8 Geography Chapter 5 Ocean Currents Additional Important Questions and Answers
Examine the following statements and correct the incorrect ones:
Question a.
Labrador is a warm ocean current.
Answer:
Incorrect.
Correct statement: Labrador is a cold ocean current.
![]()
Question b.
Surface ocean currents flow with high velocity.
Answer:
Incorrect.
Correct statement: Surface ocean currents flow with slow velocity.
Question c.
Even though the velocity of ocean currents is less, the water carried by them is immense.
Answer:
Correct.
Question d.
The ocean currents flow near the lower boundary of the continental shelf.
Answer:
Correct.
Answer the following questions in one sentence each:
Question a.
What are the main types of ocean currents?
Answer:
Cold ocean currents and warm ocean currents are the main types of ocean currents.
Question b.
In which region are the warm ocean currents formed?
Answer:
The warm ocean currents are formed in the equatorial region.
![]()
Question c.
In which regions are the cold ocean currents formed?
Answer:
The cold ocean currents are formed in the polar regions.
Question d.
Which factors are responsible for the formation of ocean currents?
Answer:
Difference in temperature of seawater, difference in density of seawater and planetary winds are the factors responsible for the formation of ocean currents.
Question e.
Which factors are responsible for the direction of flow and velocity of ocean currents?
Answer:
Rotation of the earth and continental structure are the factors responsible for the direction of flow and velocity of ocean currents.
Question f.
In which unit is the discharge of water in the oceans measured?
Answer:
The discharge of water in the oceans is measured in the Sverdrup unit.
Question g.
What is meant by 1 Sverdrup discharge?
Answer:
1 Sverdrup discharge means 1 million cu.m./second discharge of ocean water.
![]()
Question h.
Which two ocean currents meet near Newfoundland Island?
Answer:
Gulf warm ocean current and Labrador cold ocean current meet near Newfoundland Island.
Match the columns and complete the chain:
Question a.
| ‘A’ Column | ‘B’ Column | ‘C’ Column |
| 1. California Current | i. South Atlantic Ocean | a. Near the eastern coast of the continent of North America |
| 2. Hamboldt Current | ii. North Pacific Ocean | b. Near the eastern coast of the continent of South America |
| 3. Brazil Current | iii. North Atlantic Ocean | c. Near the western coast of the continent of South America |
| 4. Gulf Current | iv. South Pacific Ocean | d. Near the western coast of the continent of North America |
Answer:
| ‘A’ Column | ‘B’ Column | ‘C’ Column |
| 1. California Current | ii. North Pacific Ocean | d. Near the western coast of the continent of North America |
| 2. Hamboldt Current | iv. South Pacific Ocean | c. Near the western coast of the continent of South America |
| 3. Brazil Current | i. South Atlantic Ocean | b. Near the eastern coast of the continent of South America |
| 4. Gulf Current | iii. North Atlantic Ocean | a. Near the eastern coast of the continent of North America |
Answer the following questions in brief:
Question a.
Write in brief about warm ocean currents.
Answer:
1. Ocean currents that move water away from the Equator to the poles are called warm ocean currents.
2. Warm ocean currents are formed in the equatorial region and they flow towards the poles.
3. The warm currents increase the temperature and precipitation in the coastal areas along with which they flow. For example, the warm ocean currents flowing along with the coastal areas of Western Europe, Southern Alaska and Japan increases the temperature in the coastal areas along with which they flow. As its effect, the ports in the coastal areas of Western Europe, Southern Alaska and Japan do not freeze in winter.
4. Gulf stream, Agulhas, Somali, etc. are some of the warm ocean currents.
![]()
Question b.
Write in brief about cold ocean currents.
Answer:
1. Ocean currents that move water away from the poles to the Equator are called cold ocean currents.
2. Cold ocean currents are formed in the polar region and they flow towards the equatorial region.
3. The cold currents decrease the temperature and precipitation in the coastal areas along with which they flow. For example, the cold ocean currents flowing along with the coastal areas of Peru, Chile, and southeastern Africa, decreases the amount of precipitation in the coastal areas along with which they flow. As its effect, arid desert areas are formed in Peru, Chile, and southwestern Africa.
4. Falkland, Labrador, Canary, Oyashio, Benguela, etc. are some of the cold ocean currents.
Question c.
Write in brief about conveyor belt.
Answer:
- Due to deep ocean currents, the warm water goes down and the cold water comes to the surface of the earth.
- Thus, deep ocean currents redistribute the ocean water.
- This redistribution of ocean water takes around 500 years to complete.
- This redistribution (movement) of sea water is known as conveyor belt.
Question d.
Write in brief about relation between ocean currents and fishing.
Answer:
- Algae, plankton, etc. fish food grow on a large scale in the areas where warm ocean current and cold ocean current meet.
- Fish come in these areas on a large scale and breed. Therefore, extensive fishing
grounds are found in the meeting point of warm and cold ocean currents. - For example, Grand Bank in Atlantic Ocean near the coastal region of North America, Dogger Bank near the continent of Europe, etc.
Explain the effect of the following:
Question a.
Cold ocean currents on climate.
Answer:
- The amount of precipitation decreases in the coastal region along with which the cold ocean currents flow.
- Similarly, the temperature decreases in the coastal region along with which the cold ocean currents flow.
Give geographical reasons:
Question a.
Fog is found near Newfoundland island.
Answer:
- Fog is found at the meeting point of warm and cold ocean currents.
- Warm Gulf Stream ocean current and cold Labrador ocean current meet near Newfoundland island. Therefore, fog is found near Newfoundland island.
![]()
Question b.
Ocean transport is carried out along with ocean currents.
Answer:
- Ocean transport carried out along with ocean currents increases the speed of ocean transport and so saves the time.
- Ocean transport carried out along with ocean currents decreases the cost of fuel. Therefore, ocean transport is carried out along with ocean currents.
Question c.
Extensive fishing ground is found near Newfoundland island.
Answer:
- Warm Gulf Stream ocean current and cold Labrador ocean current meet near Newfoundland island.
- Algae, plankton, etc. fish food grow on a large scale in the meeting point of warm Gulf Stream ocean current and cold Labrador ocean current.
- Fish come in this area on a large scale and breed. Therefore, extensive fishing ground is found near Newfoundland island.
Differentiate between the following:
Question a.
Cold ocean current and Warm ocean current:
Answer:
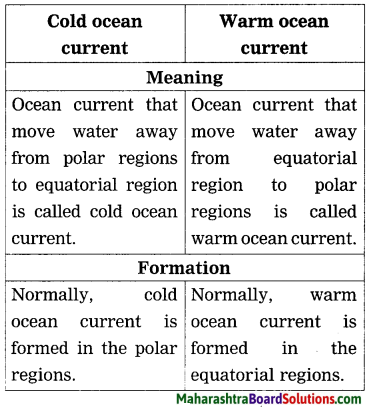
Question b.
Surface ocean current and Deep ocean current:
Answer:
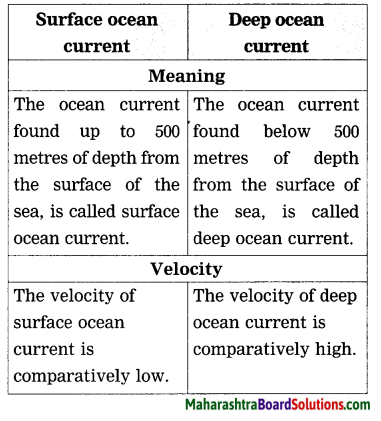
Study the following map/figure /graph and answer the following questions:
Make friends with maps!
Study the Figure and answer the following questions:

Question a.
What are the major types of ocean currents?
Answer:
Warm ocean currents and cold ocean currents are the major types of ocean currents.
Question b.
What do you call the currents flowing from the equator to the poles?
Answer:
The currents flowing from the equator to the poles are called warm currents.
![]()
Question c.
What do you call the currents flowing from the poles to the equator?
Answer:
The currents flowing from the poles to the equator are called cold currents.
Question d.
When the currents are moving in a circular manner, what difference is visible in their direction in Northern and Southern Hemisphere respectively?
Answer:
When the currents are moving in a circular manner, they will move in clockwise in Northern Hemisphere and anti-clockwise in the Southern Hemisphere.
Question e.
What might happen at the places where these two currents meet?
Answer:
Dense fog will get formed at the places where these two currents meet.
Question f.
When two different types of currents meet along the coast then what type of human settlements and occupations are seen?
Answer:
When two different types of currents meet along the coast then dense human settlements and fishing occupation are seen there.
Make friends with maps!
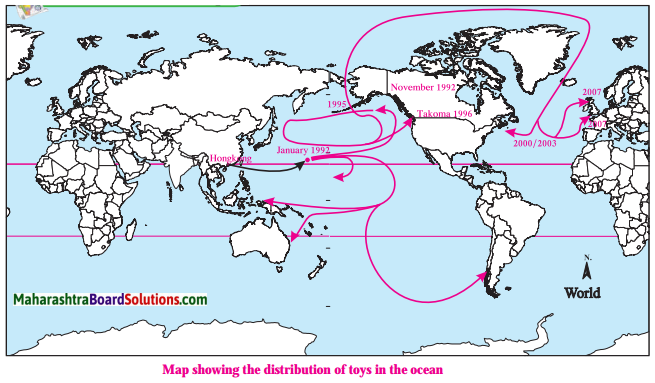
Question a.
Where did a container full of toys fall on 10th January, 1992?
Answer:
A container full of toys fell near the Hawaii Island on 10th January, 1992.
![]()
Question b.
Where did some of the toys reach on 16th November, 1992?
Answer:
Some of the toys reached the coast of Alaska on 16th November, 1992.
Question c.
Where did some of the toys reach by the year 2000?
Answer:
Some of the toys crossed the Bering Strait and reached the Arctic Ocean by the year 2000.
Question d.
Where did some of the toys reach by the year 2003?
Answer:
Some of the toys reached the eastern coast of America by the year 2003.
Question e.
Where did some of the toys reach by the year 2007?
Answer:
Some of the toys reached the western coast of continent of Europe by the year 2007.
![]()
Question f.
Why did the toys travel in this way?
Answer:
The toys travelled in this way due to surface ocean currents and mostly due to deep ocean currents.
Thought Provoking Question:
Question a.
What will be the effect of Westerlies on the ocean currents?
Answer:
- Due to the influence of the Westerlies, the ocean currents will flow from west to east in the mid-latitudes.
- Due to the influence of Westerlies, the ocean currents will flow from east to west in equatorial region. This in turn, will lead to a circular pattern of sea currents.
Open-Ended Question:
Question a.
With which points will you explain the effects of ocean currents?
Answer:
The effects of ocean currents can be explained with the help of the following points:
1. Temperature: Ocean currents brings change in the temperature of the coastal areas along with which they flow. For example, the warm ocean current flowing along the coastal areas of Japan increases the temperature in the coastal areas.
2. Precipitation: Ocean currents brings change in amount of precipitation in the coastal areas along with which they flow. For example, the cold ocean current flowing along the coastal areas of Chile decreases the precipitation in the coastal areas leading to formation of desert areas.
3. Occupation: Ocean currents also supports the growth of fishing occupation. The extensive fishing grounds redeveloped the meeting point of warm and cold ocean currents. For example, Grand Bank near the continent of North America.
4. Transportation: Sea transport carried out along with ocean currents increases the speed and reduces the cost of fuel.
8th Std Geography Questions And Answers:
- Local Time and Standard Time Class 8 Geography Questions And Answers
- Interior of the Earth Class 8 Geography Questions And Answers
- Humidity and Clouds Class 8 Geography Questions And Answers
- Structure of Ocean Floor Class 8 Geography Questions And Answers
- Ocean Currents Class 8 Geography Questions And Answers
- Land Use Class 8 Geography Questions And Answers
- Population Class 8 Geography Questions And Answers
- Industries Class 8 Geography Questions And Answers
- Map Scale Class 8 Geography Questions And Answers
- Field Visit Class 8 Geography Questions And Answers