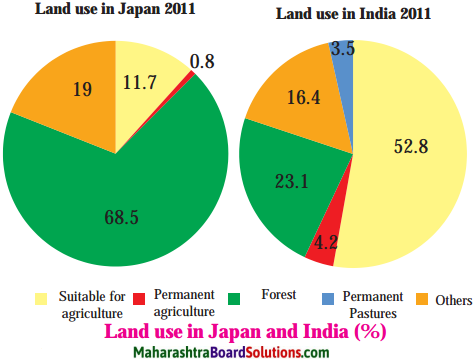
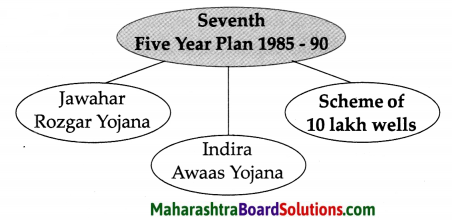
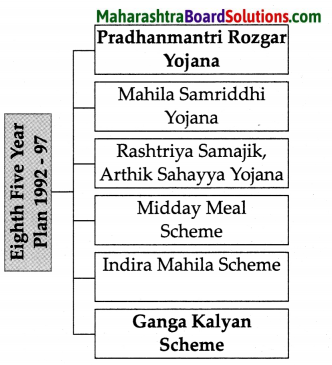
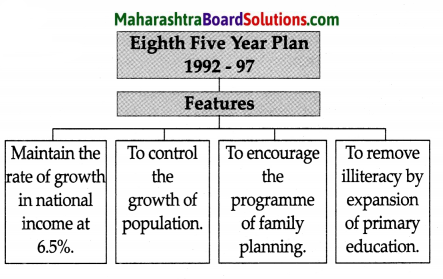
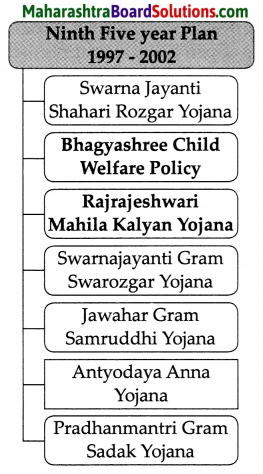
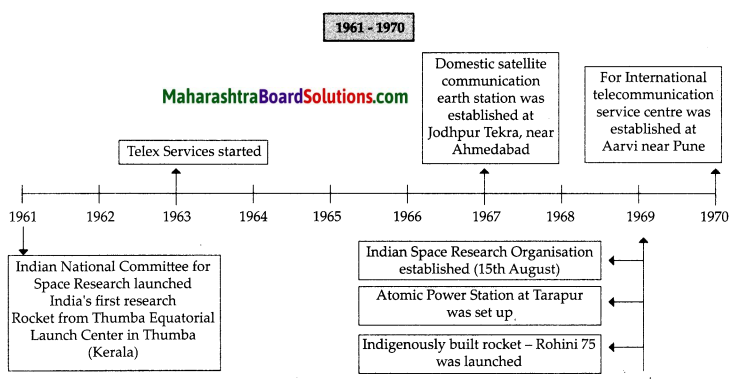
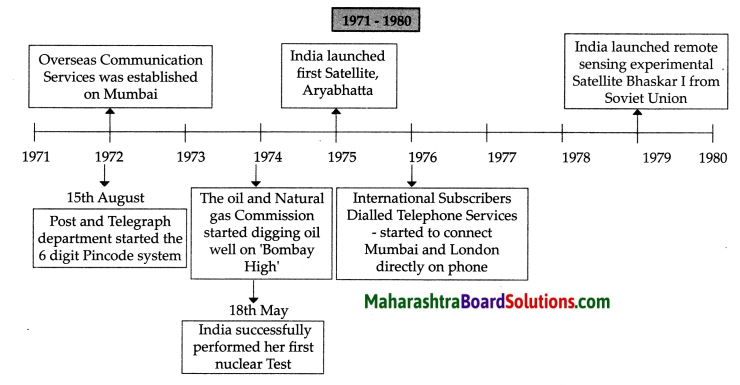
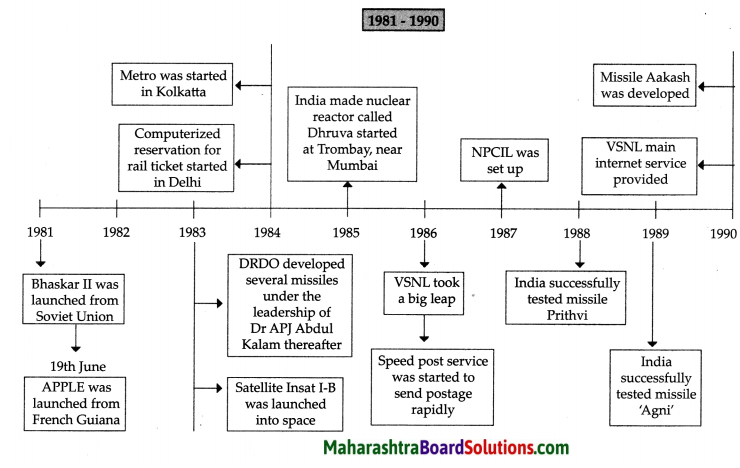
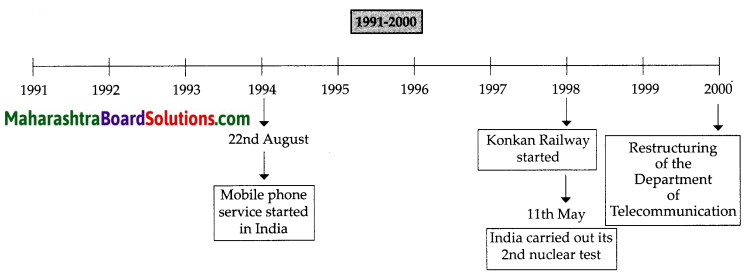
Balbharti Maharashtra State Board Class 8 Geography Solutions Chapter 3 Humidity and Clouds Notes, Textbook Exercise Important Questions and Answers.
Class 8 Geography Chapter 3 Humidity and Clouds Questions And Answers Maharashtra Board
Humidity and Clouds Class 8 Questions And Answers Chapter 3 Maharashtra Board
Class 8 Geography Chapter 3 Humidity and Clouds Textbook Questions and Answers
1. Match the column and complete the chain:
| ‘A’ Column | ‘B’ Column | ‘C’ Column |
| 1. Cirrus | i. Vertical extent in the sky | a. Roaring clouds |
| 2. Cumulonimbus | ii. Higher altitude | b. Floating clouds |
| 3. Nimbostratus | iii. Medium altitude | c. Continuous rainfall |
| 4. Alto-cumulus | iv Low altitude | d. Snowflake clouds |
Answer:
| ‘A’ Column | ‘B’ Column | ‘C’ Column |
| 1. Cirrus | ii. Higher altitude | d. Snowflake clouds |
| 2. Cumulonimbus | i. Vertical extent in the sky | a. Roaring clouds |
| 3. Nimbostratus | iv Low altitude | c. Continuous rainfall |
| 4. Alto-cumulus | iii. Medium altitude | b. Floating clouds |
2. Choose the correct word from the brackets and complete the sentences:
(Options: Cumulonimbus, relative humidity, absolute humidity, condensation, vapor-holding capacity)
Question a.
The ………….. of the air is dependent on the temperature of the air.
Answer:
vapour-holding capacity
![]()
Question b.
The amount of vapour in 1 cu.m, of air shows the ………….
Answer:
absolute humidity
Question c.
As …………. is less in the desert areas, the air is dry there.
Answer:
relative humidity
Question d.
……………. type of clouds are indicators of the storm.
Answer:
Cumulonimbus
![]()
Question e.
In a free environment, the ………….. of the vapour present in the atmosphere takes place around the dust particles.
Answer:
condensation
3. Differentiate between:
Question a.
Humidity and clouds:
Answer:
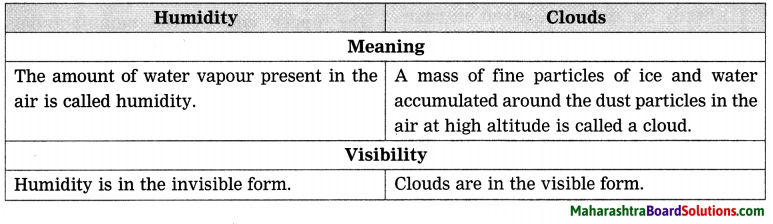
Question b.
Relative humidity and Absolute humidity
Answer:
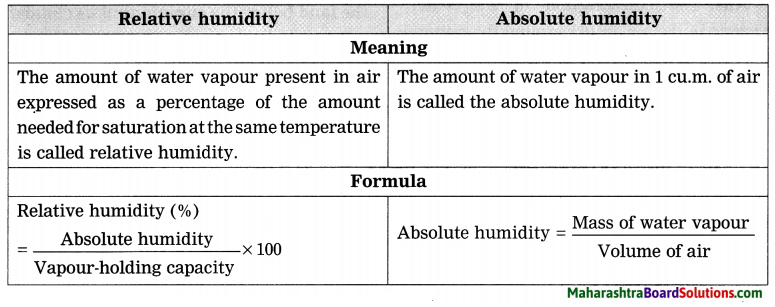
Question c.
Cumulus clouds and Cumulonimbus clouds
Answer:
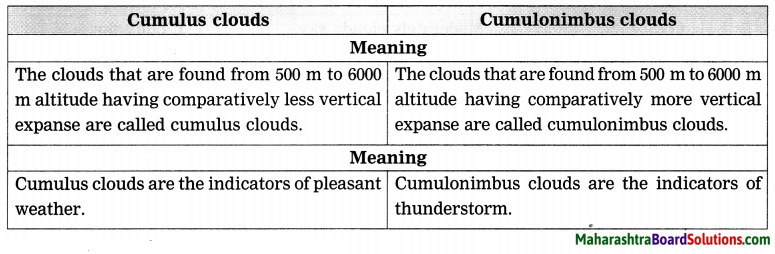
4. Answer the following questions:
Question a.
Why is the air in a region dry?
Answer:
Due to the low amount of the moisture in the air, the air in a region is I dry.
![]()
Question b.
How is humidity measured?
Answer:
1. The amount of water vapour in 1 cu.m, of air is called the absolute humidity.
2. Absolute humidity is measured with the help the following formula:
Absolute humidity
= \(\frac{\text { Mass of water vapour }}{\text { Volume of air }}\)
3. The amount of water vapour present in air expressed as a percentage of the amount needed for saturation at the same temperature is called relative humidity.
4. Relative humidity is measured with the help of the following formula: Relative humidity (%)
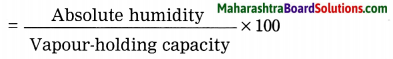
Question c.
What are the prerequisites for condensation?
Answer:
High relative humidity and dew point temperature of the air are the j prerequisites for condensation.
Question d.
What is a cloud? Write its types.
Answer:
A. Meaning:
A visible mass of fine particles of ice and water accumulated around the dust particles in the air at high altitude is called a cloud.
B. Types:
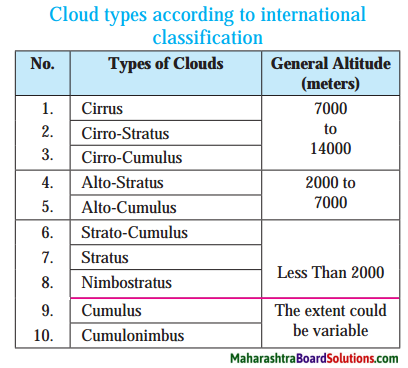
The following are the types of clouds:
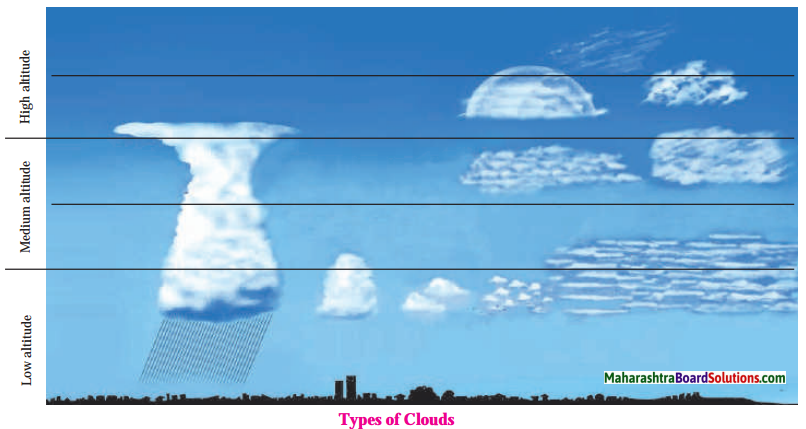
- High clouds: Clouds at an altitude of 7000 m to 14000 m are considered as high clouds. Cirrus, Cirro-Stratus and CirroCumulus are high clouds.
- Medium clouds: Clouds at an altitude of 2000 m to 7000 m are considered as medium clouds. Alto-Stratus and Alto Cumulus are medium clouds.
- Low clouds: Clouds at an altitude of less than 2000m are considered as low clouds. Strato-Cumulus, Stratus, Nimbostratus, Cumulus and Cumulonimbus are low clouds
![]()
Question e.
Which type of clouds give rain?
Answer:
Nimbostratus and cumulonimbus type of clouds give rain.
Question f.
On what does the percentage of relative humidity depend?
Answer:
The percentage of relative humidity depends on the absolute humidity and vapour-holding capacity of the air at a given temperature.
5. Give geographical reasons:
Question a.
Clouds float in the sky.
Answer:
- The condensed water or fine particles of snow accumulate around dust particles at high altitudes leading to formation of clouds.
- The condensed water or fine particles in the clouds are almost weightless. Therefore, clouds float in the sky.
Question b.
The proportion of relative humidity changes according to altitude.
Answer:
1. The temperature is found to be comparatively high in the areas at sea level. Due to high temperature, moisture-holding capacity of air near sea level is found to be high. Therefore, the humidity of the air at sea level is comparatively high.
2. The temperature is found to be comparatively low in the areas at high altitude. Due to low temperature, moisture-holding capacity of air at high altitudes is found to below. Therefore, the humidity of the air at high altitude is comparatively low. In this way, the proportion of relative humidity changes according to the altitude.
![]()
Question c.
Air becomes saturated.
Answer:
- At certain temperature, the moisture-holding capacity of air gets fulfilled and it becomes equal to the proportion of the moisture present in it.
- At this state, no more moisture can be absorbed by the air. Thus, air becomes saturated.
Question d.
Cumulus clouds change into cumulonimbus clouds.
Answer:
- Cumulus clouds are found between 500 m to 6000 m altitude. They are mostly formed due to the vertical flow of the air.
- Sometimes, the vertical expanse of the cumulus clouds increases and it leads to formation of huge mountain-like cumulonimbus clouds. Thus, cumulus clouds change into cumulonimbus clouds.
6. Solve the following:
Question a.
When the temperature of the air is 30° C, its vapour-holding capacity is 30.37 gms/cu.m., If absolute humidity is 18 gms/cu.m., then what will be the relative humidity?
Answer:
Relative humidity (%)

Question b.
What would be the absolute humidity of air if 1 cu.m, air contains 4.08 gms of vapour at 0° C temperature.
Answer:
Absolute humidity

7. Collect the weather related information from newspapers for the month of July. Relate the difference in the maximum and minimum temperatures with humidity.
Activity:
Question a.
Make a table showing the types of clouds. Use various photographs.
Answer:
Class 8 Geography Chapter 3 Humidity and Clouds Additional Important Questions and Answers
Examine the following statements and correct the incorrect ones:
Question a.
Cumulonimbus clouds are the indicators of the pleasant atmosphere.
Answer:
Incorrect.
Correct statement: Cumulonimbus clouds are the indicators of the thunderstorm.
![]()
Question b.
The relative humidity is found to be high in desert region.
Answer:
Incorrect.
Correct statement: The relative
humidity is found to be less in desert region.
Question c.
The high temperature of the air leads to rapid evaporation.
Answer:
Correct.
Question d.
When the humidity in the air is 0 gm/cu.m. at any temperature, the air is said to be humid.
Answer:
Incorrect.
Correct statement: When the humidity in the air is o gm/cu.m. at any temperature, the air Is said to be dry.
Question e.
Cirrostratus appears like a bed sheet with wrinkles.
Answer:
Correct.
Answer the following questions in one sentence each:
Question a.
What is evaporation?
Answer:
The process of converting water into steam or water vapour is called evaporation.
Question b.
What is meant by the moisture-holding capacity of air?
Answer:
The capacity of air to hold moisture at a given temperature, is called the moisture-holding capacity of air.
![]()
Question c.
What is meant by the saturation of the air?
Answer:
The condition of air at a certain temperature, in which the moisture-holding capacity of air becomes equal to the I proportion of moisture present in it, is called saturation of the air.
Answer the following questions in brief:
Question a.
Write in brief about the features of high clouds.
Answer:
Clouds at an altitude of 7000 m to 14000 m are considered as high clouds. High clouds are mainly made up of ice particles. Cirrus, Cirro-Stratus and Cirro-Cumulus are high clouds. The following are the features of high clouds :
- Cirrus: Cirrus are wispy.
- Cirro-Stratus: Cirro-Stratus look like bed sheet with wrinkles. A halo is generally seen around these clouds.
- Cirro-Cumulus clouds: Cirro- Cumulus look like groups of small waves.
Question b.
Write in brief about the features of medium clouds.
Answer:
Clouds at an altitude of 2000 m to 7000 m are considered as medium clouds. Alto-Stratus and Alto-Cumulus are medium clouds. The following are the features of medium clouds :
- Alto-Stratus : Alto-Stratus are comparatively thin. The sun is visible through these clouds as if seen through a milky glass.
- Alto-Cumulus : Alto-Cumulus are in the form of layers. They have wave-like structure. They are white in colour and have a grey shade.
Question c.
Write in brief about the features of Strato-Cumulus, Stratus and Nimbostratus.
Answer:
The features of Strato-Cumulus, Stratus and Nimbostratus are as follows :
- Strato-Cumulus: Strato-Cumulus have layers. These clouds are mostly seen in round clusters. They are white to earthy in colour.
- Stratus: Stratus are found in layers. They have uniform base. They are ash coloured.
- Nimbostratus: Nimbostratus have thick layers. They are greyish in colour. They cause continuous rainfall. They are also responsible for snowfall.
![]()
Question d.
Write in brief about the features of Cumulus and Cumulonimbus.
Answer:
The features of Cumulus and Cumulonimbus are as follows :
1. Cumulus : Cumulus are found between 500 m to 6000 m altitude. They are mostly formed due to the vertical flow of the air. They have huge size and dome-like shape. They are grey in colour. They indicate pleasant atmosphere. With an increase in the vertical expanse, these clouds turn into cumulonimbus clouds and bring rain.
2. Cumulonimbus : Cumulonimbus appear like huge mountain. They are dense and dark in colour. They have anvil-like shape at the top. They are the indicators of thunder, lightning and storm. They bring rain with storm. Sometimes they also bring hailstones. The raindrops of these clouds are found to be larger in size.
Give geographical reasons for the following:
Question a.
The absolute humidity of air in coastal region is higher than humidity of air in inland areas.
Answer:
1. The rate of evaporation is found to be high in the coastal region. As its effect, the amount of moisture in air in coastal region is found to be high. This results in high absolute humidity of air in coastal region.
2. On the other hand, the rate of evaporation is found to be low in the inland areas. As its effect, the amount of moisture in air in inland regions is found to be low. This results in low absolute humidity of air in inland areas. Thus, the absolute humidity of air in coastal region is higher than humidity of air in inland areas.
Question b.
The absolute humidity of air in equatorial region is higher than humidity of air in polar region.
Answer:
1. Equatorial region receives perpendicular sunrays throughout the year leading to high temperature. High temperature increases the rate of evaporation and this further leads to high absolute humidity of air in equatorial region.
2. Polar regions receive extremely slanted sunrays throughout the year leading to very low temperature. Very low temperature decreases the rate of evaporation and this further leads to very low absolute humidity of air in polar region. Thus, the absolute humidity of air in equatorial region is higher than humidity of air in polar region.
![]()
Question c.
Damp air is found in the coastal regions.
Answer:
- The rate of evaporation is found to be high in the coastal region. As its effect, the amount of moisture in air in coastal region is found to be high.
- The absolute humidity of the air near coastal region is found to be high. Therefore, damp air is found in the coastal regions.
Question d.
Dry air is found in the inland regions.
Answer:
- The rate of evaporation is found to be low in inland region. As its effect, the amount of moisture in air in inland region is found to be low.
- The absolute humidity of the air in inland region is found to be low. Therefore, dry air is found in inland regions.
Differentiate between the following :
Question a.
Densification and Sublimation :
Answer:

Study the following map/figure /graph and answer the following questions:
1. Study the figure 3.7 given on page 20 of the textbook and answer the following questions :
Question a.
What is the maximum diameter of the raindrop?
Answer:
The maximum diameter of the raindrop is 5 mm.
Question b.
Around what does the condensation of the water vapour in the air occur?
Answer:
The condensation of the water vapour in the air occurs around the minute particles of dust or salt in the air.
2. Study the Figure 3.9 given on page 22 of the textbook and answer the following questions :
Question a.
Which type of charge is found at the upper end of the cumulonimbus clouds?
Answer:
Positive charge is found at the upper end of the cumulonimbus clouds.
![]()
Question b.
Which type of charge is found at the low end of the cumulonimbus clouds?
Answer:
Negative charge is found at the low end of the cumulonimbus clouds.
Question c.
Which type of charge is found on the land below the cumulonimbus clouds?
Answer:
Positive charge is found on the land below the cumulonimbus clouds.
Use your brainpower!
Find where the symbols given below are used while showing the weather of a place. Write their meanings in the boxes given below:
(Note: The answer is given directly.)
Question a

Answer:
Thought-Provoking Questions:
Think about it.
Question a.
During winters, when you exhale on the glass of your mirror, what happens. If you do this in summer why doesn’t it happen?
Answer:
1. During winters, the moisture-holding capacity of air is low due to low temperature. Therefore during winters, when we exhale on the glass of mirror, the condensation of water vapour present in the exhaled air takes place. As its effect very thin water drops are found on the glass of mirror.
2. During summer, the moisture-holding capacity of air is high due to high temperature. Therefore during summer, when we exhale on the glass of mirror, the water vapour present in the exhaled air gets absorbed in air. Therefore, no water drops are found on the glass of mirror.
![]()
Give it a try.
The vapour-holding capacity of 1 cu.m, of air in various temperature is given in the following table.
Calculate the difference in the capacities by observing the following table :
(Note: The answer is given directly.)
Question a.
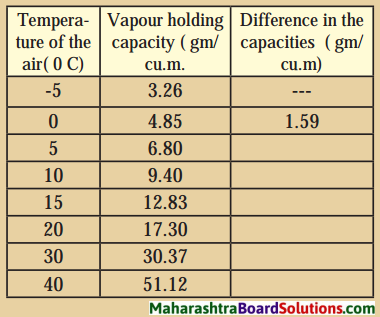
Answer:
| Temperature of the air (°C) | Vapour-holding (gm/cu.m3 (gm/cu.m3)capacity | Difference in the capacities (gm/cu.m3) |
| -5 | 3.26 | — |
| 0 | 4.85 | 1.59 |
| 5 | 6.80 | 1.95 |
| 10 | 9.40 | 2.60 |
| 15 | 12.83 | 3.43 |
| 20 | 17.30 | 4.47 |
| 30 | 30.37 | 13.07 |
| 40 | 51.12 | 20.75 |
Use your brain power!
Question a.
In which season is humidity generally more?
Answer:
Humidity is generally more in monsoon.
Question b.
How does the humidity affect the human body?
Answer:
The rate of respiration and sweating increases due to humidity.
Question c.
Observe how humidity affects the food materials at our home and write about the same.
Answer:
Due to humidity, fungus flourish on food materials at our home and it gets spoiled.
![]()
Question d.
Is there any relationship between formation of fungus and humidity?
Answer:
There is a direct relationship between formation of fungus and humidity. Humidity supports the growth of fungus.
Question e.
How is the early or late drying up of clothes related to humidity?
Answer:
As the moisture-holding capacity is found to be high in low humidity air, it leads to early drying up of clothes. As the moisture-holding capacity is found to be low in high humidity air, it leads to late drying up of clothes.
Think about it.
Question a.
What will happen if the temperature of saturated air at 20° C drops to 10° C abruptly?
Answer:
- If the temperature of saturated air at 20° C drops to 10° C abruptly, the relative humidity of the air will increase abruptly.
- Rise in the relative humidity will lead to condensation or sublimation of water vapour in the air. This in turn, will lead to precipitation or snowfall.
![]()
Open-Ended Question:
Question a.
Explain the effect of humidity on human life.
Answer:
- High humidity increases the rate of sweating and respiration. This adversely affects the daily functioning.
- Low humidity with pleasant atmosphere, have favourable effects on the daily routine.
- High humidity leads to late drying up of clothes and early spoiling of food materials.
- Low humidity leads to early drying up of clothes and late spoiling of food materials.
8th Std Geography Questions And Answers:
- Local Time and Standard Time Class 8 Geography Questions And Answers
- Interior of the Earth Class 8 Geography Questions And Answers
- Humidity and Clouds Class 8 Geography Questions And Answers
- Structure of Ocean Floor Class 8 Geography Questions And Answers
- Ocean Currents Class 8 Geography Questions And Answers
- Land Use Class 8 Geography Questions And Answers
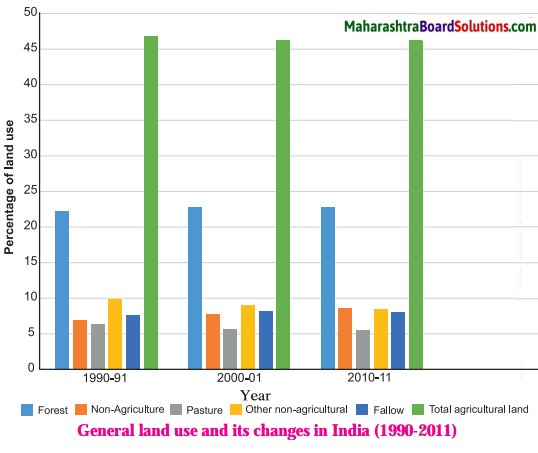
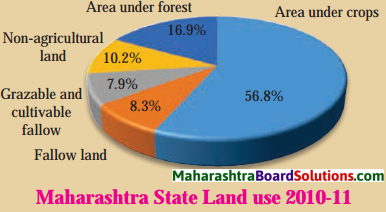
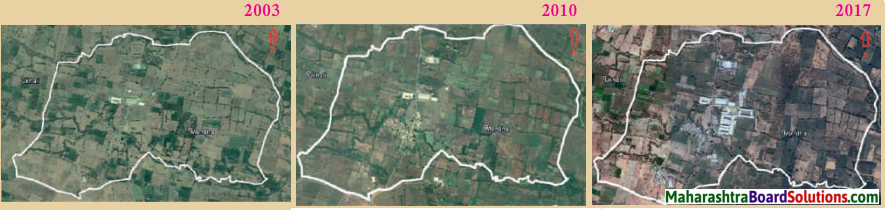
- Population Class 8 Geography Questions And Answers
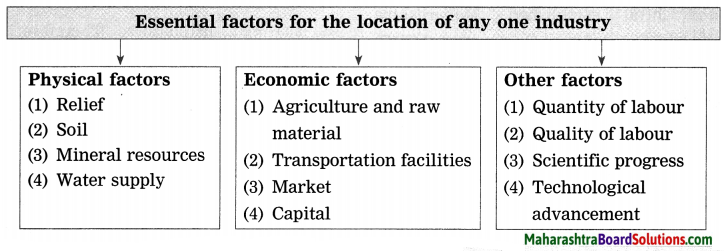
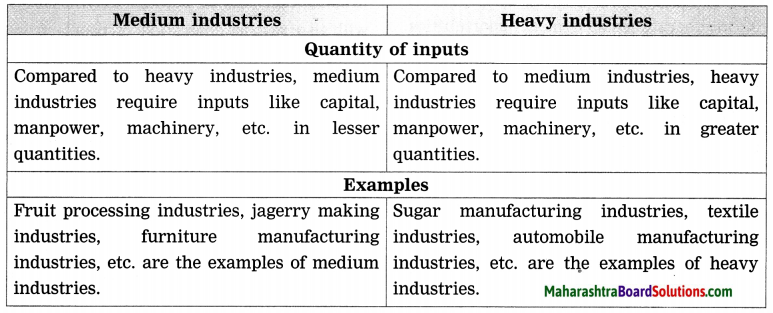
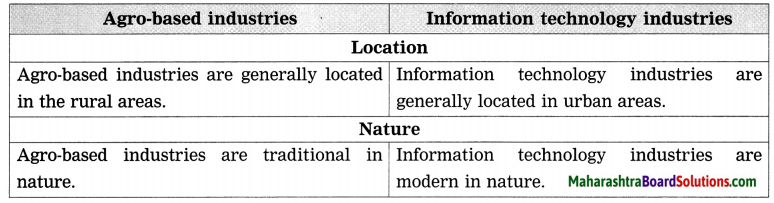
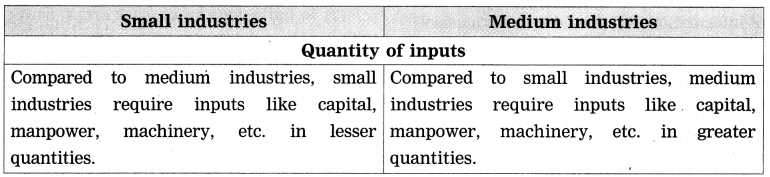
- Industries Class 8 Geography Questions And Answers
- Map Scale Class 8 Geography Questions And Answers
- Field Visit Class 8 Geography Questions And Answers