Balbharti Maharashtra State Board Class 8 Science Solutions Chapter 12 Introduction to Acid and Base Notes, Textbook Exercise Important Questions and Answers.
Std 8 Science Chapter 12 Introduction to Acid and Base Question Answer Maharashtra Board
Class 8 Science Chapter 12 Introduction to Acid and Base Question Answer Maharashtra Board
1. Identify the following solutions, whether they are acid or base.
Question a.
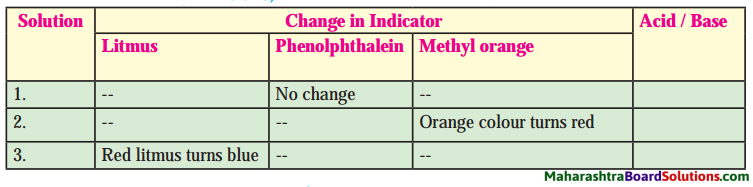
Answer:
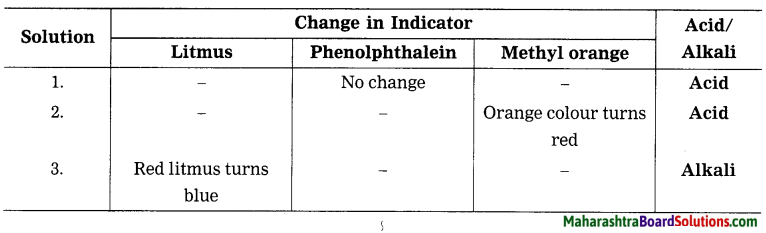
2. Write chemical names from given formulae.
H2SO4, Ca(OH)2, HCl, NaOH, KOH, NH4OH
Question a.
Write the chemical names from given formulae: H2SO4, Ca(OH)2, HCl, NaOH, KOH, NH4OH
Answer:
- H2SO4 – Sulphuric acid
- Ca(OH)2 – Calcium hydroxide
- HCl – Hydrochloric acid
- NaOH – Sodium hydroxide
- KOH – Potassium hydroxide
- NH4OH – Ammonium hydroxide
![]()
3. Sulphuric acid has highest importance in chemical Industry. Why?
Question a.
Sulphuric acid has highest importance in chemical Industry. Why?
Answer:
Answer:
Sulphuric acid has highest importance in the chemical industry because it is used in the manufacturing of fertilizers like ammonium sulphate and superphosphate of lime. It is used in the manufacturing of hydrochloric acid, nitric acid, phosphoric acid, ether, plastics and metal sulphates. It is also used in the manufacturing of dyes, drugs, perfumes, disinfectants and glue.
4. Give answers.
Question a.
Which acid is used for getting chloride salt?
Answer:
Hydrochloric acid is used for getting chloride salt.
Question b.
By squeezzing lemon on a piece of rock the gas liberated turned lime water milky. Which compound is present in the rock?
Answer:
Metal carbonate is present in the rock.
Question c.
The label on the bottle of chemical is spoiled. How will you find whether the chemical is acidic or not?
Answer:
The chemical is tested with blue litmus paper. If it turns red it is an acidic otherwise tested with red litmus paper, if it turns blue, it is an alkaline.
![]()
5. Answer the following questions.
Question a.
Explain the difference between acid and base.
Answer:
Acids:
- Acids have sour taste.
- Acids turn blue litmus red.
Bases:
- Bases have bitter taste.
- Bases turn red litmus blue.
Question b.
Why indicator does not affect by salt?
Answer:
Mostly indicators are organic compounds which do not react with a salt.
Question c.
Which substances are produced i by neutralization process?
Answer:
Salt and water are produced in the f neutralization process.
![]()
Question d.
Which are the industrial uses of acids?
Answer:
Acids which are used in the industry are:
- Sulphuric acid
- Hydrochloric acid
- Nitric acid.;
1. Sulphuric acid: Sulphuric acid is 5 used (a) in the manufacture of chemical? fertilizers like ammonium sulphate, (b) for cleaning gold and silver articles and (c) in car batteries.
2. Hydrochloric acid: Hydrochloric acid is used (a) to clean toilets and (b) to obtain glucose from starch and also for producing gelatine.
3. Nitric acid: Nitric acid is used (a) in the manufacture of perfumes (b) for engraving on copper, brass or silver and (c) in the manufacture of paints and explosives.
6. Select proper word given in bracket and fill in the blanks.
Question a.
Main constituent of acid is ……………. .
Answer:
Main constituent of acid is H+ ion.
Question b.
Main constituent of alkali is …………….. .
Answer:
Main constituent of alkali is OH– ion.
Question c.
Tartaric acid is a …………… acid.
Answer:
Tartaric acid is a weak acid.
![]()
7. Match the pairs.
Question a.
| Group A | Group B |
| 1. Tamarind | a. Acetic acid |
| 2. Curd | b. Citric acid |
| 3. Lemon | c. Tartaric acid |
| 4. Vinegar | d. Lactic acid |
Answer:
| Group A | Group B |
| 1. Tamarind | c. Tartaric acid |
| 2. Curd | d. Lactic acid |
| 3. Lemon | b. Citric acid |
| 4. Vinegar | a. Acetic acid |
8. State true or false.
Question a.
Oxides of metals are alkaline in nature.
Answer:
True.
Question b.
Salt is acidic.
Answer:
False. (Salt is neutral)
Question c.
Metal corrodes due to salts.
Answer:
False. (Acids and bases corrode metals)
Question d.
Salts are neutral.
Answer:
True. (Not all salts neutral)
![]()
9. Classify the following substances into acidic, basic and neutral group:
HCl, NaCl, MgO, KCl, CaO, H2SO4, HNO3, H2O and Na2CO3.
Question a.
Classify the following substances into acidic, basic and neutral group:
HCl, NaCl, MgO, KCl, CaO, H2SO4, HNO3, H2O and Na2CO3.
Answer:
| Group | Substances |
| Acid | HCl, H2SO4, HNO3 |
| Base | CaO, MgO, Na2CO3 |
| Neutral | H2O, NaCl, KCl. |
Project:
Question a.
Write in your own language the uses and importance of neutralization reaction in daily life.
Class 8 Science Chapter 12 Introduction to Acid and Base Important Questions and Answers
Fill in the blanks:
Question 1.
Acid reacts with metal to form …………. gas.
Answer:
Acid reacts with metal to form hydrogen gas.
Question 2.
DNA is an acid present in our body, it decides …………. properties.
Answer:
DNA is an acid present in our body, it decides heredity properties.
Question 3.
The chemical formula of milk of magnesia is ………….
Answer:
The chemical formula of milk of magnesia is Mg (OH)2.
Question 4.
…………. are used to control hyperacidity.
Answer:
Antacids are used to control hyperacidity.
Question 5.
Alkali has …………. taste.
Answer:
Alkali has bitter taste.
Question 6.
Proteins are made up of ………….
Answer:
Proteins are made up of amino acids.
Question 7.
…………. is used in batteries.
Answer:
Dil. H2SO4 is used in batteries.
Rewrite the following statements by selecting the correct options:
Question 1.
The colour of phenolphthalein indicator in alkaline solution is ………….
(a) yellow
(b) green
(c) orange
(d) pink
Answer:
The colour of phenolphthalein indicator in alkaline solution is pink.
Question 2.
…………. is sour to taste.
(a) An acid
(b) An alkali
(c) A salt
(d) Alcohol
Answer:
An acid is sour to taste.
Question 3.
When phenolphthalein is added to NaOH, the colour of the solution will become ……………… .
(a) colourless
(b) red
(c) pink
(d) yellow
Answer:
When phenolphthalein is added to 5 NaOH, the colour of the solution will become pink.
Question 4.
When phenolphthalein is added to HCl, the colour of the solution will be ………….. .
(a) red
(b) pink
(c) green
(d) colourless
Answer:
When phenolphthalein is added to HCl, the colour of the solution will be colourless.
Question 5.
…………. is a natural indicator.
(a) Phenolphthalein
(b) Methyl orange
(c) Litmus
(d) Methyl red
Answer:
Litmus is a natural indicator.
Question 6.
The litmus paper or the litmus solution is obtained from …………. plants.
(a) moss
(b) rose
(c) hibiscus
(d) lichen
Answer:
The litmus paper or the litmus solution is obtained from lichen plants.
Question 7.
…………. is not an alkali.
(a) Sodium hydroxide
(b) Potassium hydroxide
(c) Copper hydroxide
(d) Calcium hydroxide
Answer:
Copper hydroxide is not an alkali.
Question 8.
…………. is a weak acid.
(a) Hydrochloric acid
(b) Nitric acid
(c) Carbonic acid
(d) Sulphuric acid
Answer:
Carbonic acid is a weak acid.
Question 9.
When methyl orange is added to HCl, the colour of the solution will be …………… .
(a) red
(b) pink
(c) yellow
(d) colourless
Answer:
When methyl orange is added to HCl, the colour of the solution will be pink.
Question 10.
When blue litmus paper is added to NaOH, the colour of the litmus paper will be ……………. .
(a) blue
(b) red
(c) pink
(d) yellow
Answer:
When blue litmus paper is added to NaOH, the colour of the litmus paper will be blue.
State whether the following statements are true or false. If a statement is false, correct it and rewrite:
Question 1.
Red cabbage is a natural indicator.
Answer:
True
Question 2.
Fats of our body are formed by fatty acids.
Answer:
True.
Question 3.
Ammonium hydroxide is used in the production of fertilizers.
Answer:
True.
Question 4.
Lime water is a weak acid.
Answer:
False. (Limewater is a weak base)
Question 5.
Orange colour of methyl orange turns yellow in acid.
Answer:
False. (Orange colour of methyl orange turns pink in acid)
Question 6.
Methyl red turns yellow in alkali.
Answer:
True.
Question 7.
Oxalic acid is used in aerated cold drinks.
Answer:
False. (Carbonic acid is used in aerated cold drinks).
Find the odd one out and justify:
Question 1.
Acetic acid, carbonic acid, hydrochloric acid, nitric acid.
Answer:
Acetic acid. (Others are mineral acids.)
Question 2.
Hydrogen chloride, sodium hydroxide, calcium oxide, ammonia.
Answer:
Hydrogen chloride. (Others are bases.)
Question 3.
HCl, CH3COOH, H2SO4, HNO3
Answer:
CH3COOH. (Others are strong acids.)
Question 4.
NaOH, Ca(OH)2, NH4OH, Ba(OH)2
Answer:
NaOH (Others are weak bases.)
Question 5.
H2SO4, H2CO3, HCl, HNO3
Answer:
H2CO3 (Others are strong acids.)
Question 6.
Citric acid, formic acid, lactic acid, nitric acid.
Answer:
Nitric acid. (Others are organic acids.)
Question 7.
Lime, litmus, phenolphthalein, methyl orange.
Answer:
Lime. (Others are indicators.)
Match the following:
Question 1.
| Column ‘A’ | Column ‘B’ |
| 1. Strong acid | a. Magnesium hydroxide |
| 2. Weak alkali | b. Carbonic acid |
| 3. Weak acid | c. NaOH |
| 4. Strong alkali | d. Nitric acid |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Strong acid | d. Nitric acid |
| 2. Weak alkali | a. Magnesium hydroxide |
| 3. Weak acid | b. Carbonic acid |
| 4. Strong alkali | c. NaOH |
![]()
Question 2.
| Column ‘A’ | Column ‘B’ |
| 1. Sodium hydroxide | a. Whitewashing |
| 2. Magnesium hydroxide | b. Fertilizers |
| 3. Calcium hydroxide | c. Washing soap |
| 4. Ammonium hydroxide | d. Antacid |
Answer:
| Column ‘A’ | Column ‘B’ |
| 1. Sodium hydroxide | c. Washing soap |
| 2. Magnesium hydroxide | d. Antacid |
| 3. Calcium hydroxide | a. Whitewashing |
| 4. Ammonium hydroxide | b. Fertilizers |
![]()
Define the following:
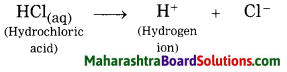
1. Acid: A substance which gives H+ ions in water is called an acid.
2. Alkali: A substance which gives OH– ions in water is called an alkali.
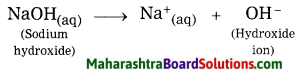
3. Indicator: Substances which change their colours in presence of acid or base are called indicators.
4. Neutralization: The reaction between an acid and an alkali to form the r salt and water is termed as neutralization.
Answer the following questions in one sentence each:
Question 1.
What are natural acids?
Answer:
Acids present in foodstuffs are called natural acids.
Question 2.
State three vegetables from which natural indicators can be prepared.
Answer:
Natural indicators: Red cabbage, radish, tomato.
![]()
Question 3.
State names of any three strong acids.
Answer:
- Hydrochloric acid
- Sulphuric acid
- Nitric acid.
Question 4.
State the names of the acids found in lemon juice, Amla, tamarind water and curd.
Answer:
- Lemonuice – citric acid
- Amla – ascorbic acid
- tamarind water – tartaric acid
- curd – lactic acid.
Question 5.
State names of any three alkalis.
Answer:
- sodium hydroxide
- potassium hydroxide
- calcium hydroxide.
Question 6.
State the names of three indicators.
Answer:
- Litmus paper
- Methyl orange
- Phenolphthalein.
![]()
Question 7.
State the names of three weak alkalis.
Answer:
- Calcium hydroxide
- ammonium hydroxide
- magnesium hydroxide.
Question 8.
From which plant is litmus obtained?
Answer:
Litmus is obtained from plants called lichens.
Question 9.
Name the antacid which is used to control the hyper acidity.
Answer:
Milk of magnesia [Mg(OH)2] is used to control the hyper acidity.
![]()
Question 10.
How will you neutralize the excess of acid present in the soil?
Answer:
Lime stone or lime water is mixed in the soil to neutralize the excess of acid.
Question 11.
When lemonuice falls on a marble kitchen counter, which is the gas that bubbled out?
Answer:
Carbon dioxide.
Answer the following questions:
Question 1.
What are indicators?
Answer:
Substances which change their colours in presence of acid or base are called indicators.
Question 2.
State two acid-base indicators and mention their colour change.
Answer:
Phenolphthalein and methyl orange are two acid-base indicators. Phenolphthalein is colourless in an acidic solution while it turns pink in a basic solution. Methyl orange gives orange colour with an acidic solution and yellow colour with a basic solution.
Question 3.
State the colour change for each of the following solutions with red litmus, blue litmus, phenolphthalein and methyl orange solutions.
Answer:
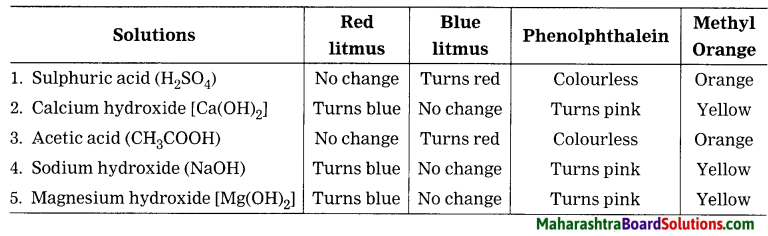
Question 4.
Name the acid present in (1) Orange (2) Vinegar (3) Tamarind (4) Curd (5) Tomato (6) Lemon.
Answer:
| Substance | Acid |
| Orange | Citric acid |
| Vinegar | Acetic acid |
| Tamarind | Tartaric acid |
| Curd | Lactic acid |
| Tomato | Oxalic acid |
| Lemon | Citric acid |
![]()
Question 5.
Classify the following into acidic substances, basic substances and salts.
(1) Lime water, (2) soda water, (3) soap water, (4) sea water, (5) sweet limeuice, (6) sugar caneuice, (7) buttermilk, (8) a mixture of ash in water, (9) tamarind water.
Answer:
a. Acidic substances: (1) Soda water, (2) sweet limeuice, (3) buttermilk, (4) tamarind water.
b. Basic substances: (1) Lime water, (2) Soap water, (3) a mixture of ash in water.
c. Salts: (1) Sea water, (2) sugar caneuice.
Question 6.
What happens when sour substances like limeuice, tamarind water falls on shahabad stones or kitchen platform? Why?
Answer:
When sour substances like limeuice, tamarind water falls on shahabad stones or kitchen platform, citric acid present in limeuice and tartaric acid in tamarind water reacts with metal carbonates present in shahabad stones or kitchen platform, carbon dioxide gas is evolved resulting in the formation of uneven surface.
Question 7.
Collect soil samples from your surroundings and find out whether it is acidic or alkaline or neutral?
Answer:
Soil samples collected from the surroundings are acidic in nature.
Question 8.
Which substances are used to clean greenish stains on copper vessels and to shine blackish silver utensils?
Answer:
Tamarind pulp is used to clean greenish stains on copper vessels. To shine blackish silver utensils, ammoniacal solution or limeuice or detergent is used.
![]()
Question 9.
Why toothpaste is used for brushing teeth?
Answer:
- Toothpaste contains fluorides and alkali to neutralize the mouth acid.
- Hence, the tooth paste, which is generally alkaline, is used for cleaning the teeth as it can neutralize the excess acid in i the mouth and prevent tooth decay.
Question 10.
State the properties of acids.
Answer:
- Acids are sour in taste.
- Acid molecules contain hydrogen ion (H+) as a main constituent.
- Acid reacts with metal to form hydrogen gas.
- Acid reacts with carbonates and liberates CO2 gas.
- Blue litmus turns red in acid.
Question 11.
State the uses of acids.
Answer:
- Acids are used in the production s of chemical fertilizers.
- Acids are used in the production of explosives, oil purification, medicines, dyes and paints.
- Hydrochloric acid is used for the preparation of different types of chloride salts.
- Dil. H2SO4 acid is used in the batteries (electric cell).
- Dil. HCl is used for sterilization of water.
- Acid is used for making of white paper from wood pulp.
![]()
Question 12.
The iron knife shines better after cutting the sour fruits like lemon, raw mangoes. Why?
Answer:
Acids present in sour fruits clean and dissolves the salts present on the surface of iron knife as a result iron knife shines better after cutting the sour fruits.
Question 13.
What is meant by neutralization? Give example.
Answer:
When an acid reacts with an alkali to form a salt and water, it is called neutralization.
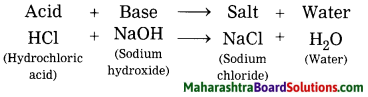
Give scientific reasons:
Question 1.
When we have stomachache, doctors prescribe medicine containing magnesium hydroxide.
Answer:
- There is hydrochloric acid in our stomach which helps in the digestion of food.
- Sometimes in the stomach, there is more hydrochloric acid than we need.
- This excess acid causes acidity and indigestion resulting in stomachache.
- When magnesium hydroxide is administered it neutralizes the extra acid in the stomach.
- Therefore, magnesium hydroxide is the best cure for stomachache.
- So, when we have stomachache, doctors prescribe medicine containing magnesium hydroxide.
Question 2.
Quicklime or slaked lime is added to the soil.
Answer:
- When chemical fertilizers are added to the soil, the soil becomes acidic.
- To reduce its acidity quicklime or slaked lime is added to the soil.
![]()
Question 3.
Tarnished and stained copper vessels are brought to a shine by using tamarind pulp.
Answer:
In due course, unus6d copper vessels
are covered with a coating of black copper oxide and other compounds of copper which tarnish the vessel. Tamarind contains tartaric acid. When the vessel is rubbed with tamarind pulp, tartaric acid reacts with copper oxide and removes the layer. Thus, the copper vessel regains its shine.
Question 4.
Copper and brass utensils are tinned.
Answer:
Copper and brass reacts with i organic acids present in food materials and poisonous salts are formed. Tin does not react with organic acids present in food materials. Therefore, copper or brass vessels are tinned.
Distinguish between acid and alkali. OR Distinguish between the following:
Question 1.
Acid and Alkali:
Answer:
| Acid | Alkali |
| 1. Acid has sour taste. | 1. Alkali has bitter taste. |
| 2. Acid turns blue litmus red. | 2. Alkali turns red litmus blue. |
| 3. An acid in an aqueous solution gives H+ ions. | 3. An alkali in an aqueous solution gives OH+ ions. |
| 4. Oxides of non – metals form acids. | 4. Oxides of metals form bases. |
![]()
Activity-based questions:
Activity 1:
Apparatus: Hibiscus, rose, turmeric, red cabbage leaves, filter paper, etc.
Activity: Rub red petals of hibiscus flower on the white filter paper. This gives hibiscus indicator paper. Similarly rub, rose petals on the white filter paper. Cut strips of this paper, it is a rose indicator paper. Take turmeric powder, add a little water in it. Dip filter paper or ordinary paper in the turmeric water for some time.
After drying make strips of that paper. Prepare turmeric indicator paper in this way. Put leaves of red cabbage in small quantity of water and heat it. Once solution of cabbage leaves cool down, dip papers in it and dry it. Make strips of dried paper. In this way prepare red cabbage indicator paper. Put some drops of following substances on the indicator papers prepared by the above method and write the effect in the following table:
| Substance | Effect on turmeric paper | Acidic/basic |
| 1. Lime juice | Yellow | Acidic |
| 2. Lime water (calcium hydroxide) | Red | Basic |
| 3. |
![]()
Activity 2:
Take baking powder. Add a little water to it. Add this solution on to limeuice, vinegar, orangeuice, appleuice, etc. and note the findings.
What do you observe on addition of baking soda solution in the fruituice? Whether bubbles formed or effervescence came out of fruituice?
From the above first activity we came to know that yellow turmeric indicator paper’s turns red in certain solutions. Similarly on addition of baking soda solution in the acidic solution bubbles come out or effervescence is produced.
By these simple and easy activity we can identify acidic or alkaline substance:
[Note for Activity 1 and Activity 2: Students should perform the experiments under the guidance of school teachers and record their observations.]
![]()
Activity 3:
Under the guidance of teacher take limeuice, ammonium hydroxide (NH4OH), dil. hydrochloric acid (dil. HCl) and nitric acid (HNOs) in different test-tubes. Add drops of following indicators in them. Also dip litmus papers in the solutions. Observe and record in the following table.
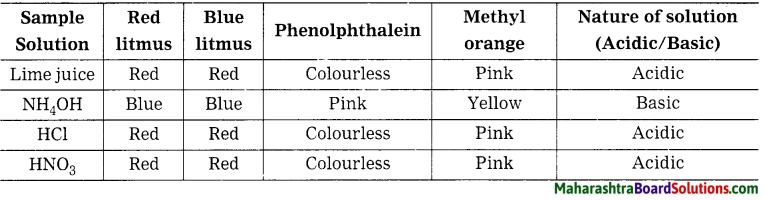
8th Std Science Questions And Answers:
- Human Body and Organ System Class 8 Questions And Answers
- Introduction to Acid and Base Class 8 Questions And Answers
- Chemical Change and Chemical Bond Class 8 Questions And Answers
- Measurement and Effects of Heat Class 8 Questions And Answers
- Sound Class 8 Questions And Answers
- Reflection of Light Class 8 Questions And Answers
- Man-made Materials Class 8 Questions And Answers
- Ecosystems Class 8 Questions And Answers
- Life Cycle of Stars Class 8 Questions And Answers
