Balbharti Maharashtra State Board 12th Chemistry Important Questions Chapter 16 Green Chemistry and Nanochemistry Important Questions and Answers.
Maharashtra State Board 12th Chemistry Important Questions Chapter 16 Green Chemistry and Nanochemistry
Question 1.
Define the following:
a. Atom economy.
Answer:
Atom economy : Atom economy is a measure of the amount of atoms from the starting materials that are present
in the final product at the end of chemical process.
![]()
Question 2.
How will you prevent the generation of waste or by-products?
Answer:
To prevent generating waste, there is the need to develop the zero waste technology (ZWT). ZWT in a chemical synthesis should result in waste product being zero or minimum. To use the waste product of one system as the raw material for other system is also the aim of ZWT.
For example :
- Cement and brick industry can use the bottom ash of thermal power station as the raw material.
- Thermal power station can use the effluent coming out from cleansing of machinery parts as coolant water.
Question 3.
(1) Calculate the atom economy of the following:
(At mass of C = 12, 11 = 1 ,0 = 16)
![]()
Answer:
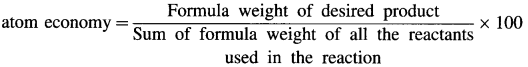
Formula weight of ethanol = 46
ethene 28
water= 18
% atom economy = \(\frac{28}{46}\) x 100 = 60.9%
(2) Calculate the atom economy of fermentation of sugar (glucose) to ethanol.
![]()
Answer:
![]()
Formula wt of glucose = 180
Formula Wi of ethanol =46
Relative massiwt of desired useful product in thc equation = 2 x 46 =92
% Atom economy = 92/180 x 100 = 51.1%
![]()
Question 4.
ExpLain less hazardous chemical synthesis with suitable example.
Answer:
- To avoid formation of hazardous waste from chemical processes, the chemical reactions and synthesis routes should be designed to be as safe as possible.
- Earlier used insecticide DDT (Dichlorodiphenyltrichloroethanc) was found to be harmful for human beings. So DDT has been replaced by benzenc hexachioride (BHC) as an insecticide, one of the y-isomer (gamma) of BHC is called gammexane or lindane.
Question 5.
How will you develop products that are less toxic or which require less toxic raw materials ?
Answer:
- There is a need to design safer chemicals to prevent the workers in chemical industries from being exposed to toxic environment.
- Adipic acid is extensively used in polymer industry. In synthesis of adipic acid, benzene is used as the starting material, but benzene is carcinogenic and being volatile organic compound (VOC) it pollutes the air and environment.
- To overcome this health hazard Green technology developed by Drath and Frost, adipic acid is enzymatically synthesised from glucose.
Question 6.
How to apply the principle of green chemistry to achieve energy efficiency?
Answer:
- Energy requirements during chemical synthesis is huge. To minimize the energy use it is better to carry out reactions at room temperature and pressure.
- This can be achieved by applying the principle of green chemistry i.e. use of catalyst, use of micro-organisms or biocatalyst and use of renewable materials, etc.
- The use of less energy can be achieved by improving the technology of heating system, use of microwave, etc.
Question 7.
Explain the use of renewable feed stocks.
Answer:
- Industries use a lot of non-renewable feed stocks like petroleum. These resources are depleting fast and the future generation will be deprived. The excessive use of these resources have also put a burden on the environment.
- If renewable resources like agricultural or biological products are used, this will ensure the sharing of resources by future generations. This practice will also not put a burden on the environment.
- The products and waste produced are generally biodegradable and environmental friendly hence leading to a sustainable future.
Question 8.
Explain the need to design degradable chemicals.
Answer:
- Environment protection is the prime concern which has lead to the need for designing chemicals that degrade and can be discarded easily. These chemicals and their degradation products should be non-toxic, non-bioaccumulative or should not be environmentally persistent.
- This principle aims at waste product being automatically degradable to clean the environment. Thus the preference for biodegradable polymers and pesticides.
- To make the separation and segregation easier for the consumer an international plastic recycle mark is printed on larger items.
Question 9.
Define the role of real time analysis in pollution prevention.
Answer:
- There is a dire need to develop improvised analytical methods to allow for real time, in process monitoring and control prior to the formation of hazardous substances.
- It is very much important for the chemical industries and nuclear reactors to develop or modify analytical
methodologies so that continuous monitoring of the manufacturing and processing unit is possible.
![]()
Question 10.
Define the role of safer chemistry in accident prevention.
Answer:
(1) It is needed to develop chemical processes that are safer and minimize the risk of accidents. It is important to select chemical substances used in a chemical reaction in such a way that they can minimize the occurrence of chemical accidents, explosions, fire and emissions.
(2) For example : Chemical process that works with the gaseous substances can lead to relatively higher possibilities of accidents including explosion as compared to the system working with nonvolatile liquid and solid substances.
Question 11.
Green chemistry plays an important role in sustainable development. Explain.
Answer:
Sustainable development is a development that protects the environment and the world’s resources. We can achieve sustainable development by adapting the twelve principles of green chemistry.
Green chemistry designs safer chemicals which are less toxic. It normally leads to low cost, use of less energy, environmentally friendly solvents and less production of waste. Green chemistry works on the principle of atom economy and minimum or no waste production. It encourages the use of renewable feed stocks and reduces the use of toxic and hazardous chemicals. It eliminates majorly stoichiometry reactions and prefers to use catalysis. It preserves the environment and safety requirements with added benefit of cost reduction.
Question 12.
How are nanomaterials classified ?
Answer:
Nanoparticles, nanowires and nanotubes can be classified according to dimensions. The nano structured materials may be large organic molecules, inorganic cluster compounds and metallic or semiconductor particles.
Question 13.
What are zero, one and two dimensional nanoscale system ?
Answer:
- Zero-Dimensional Nanostructures : A zero-dimensional structure is one in which all three dimensions are in the nanoscale.
For example : Nanoparticles. - One-Dimensional Nanostructures : A one-dimensional nanostructure is one in which two dimensions are in the nanoscale. For example : Nanowires and Nano rods.
- Two-Dimensional Nanostructures : A two-dimensional nanostructure is one in which one dimension is in the nanoscale. For example : Thin films.
Question 14.
State the different characteristic features of nanoparticles.
Answer:
The nanoparticle science is special as at such a small scale, different laws dominate than what we experience in our everyday life.
The characteristic features like optical properties, catalytical activities, have huge surface area and good thermal properties mechanical strength electrical conductivity vary than that of bulk material.
(1) Colour : At nanoscale this optical property behaves differently. Elemental gold has nice shining yellow colour, but nanoparticles of gold show red colour.
(2) Catalytic activity : Since the surface area of nanoparticles is large they show increased catalytic activity. They are usually heterogenous catalyst that means catalysts are solid form and the reactions occur on the surface of the catalyst. These catalysts can be easily separated and recycled. For example : Pd, Pt metal nanoparticles used in hydrogenation reactions. Ti02, ZnO are used in photocatalysis. Gold in bulk is unreactive but the nanoparticles of gold behave as very good catalyst for organic reactions.
(3) Surface area : High surface-to-volume ratio is a very important characteristic of nanoparticles. Bulk material if subdivided into a group of individual nanoparticles, the total volume remains the same, but the collective surface area is largely increased. With large surface area for the same volume, these small particles react much faster because more surface area provides more number of reaction sites, leading to more chemical reactivity. Explanation of increase in surface area with decrease in particle size.
(4) Thermal strength : The melting point of nanomaterial changes drastically with size.
For example : Sodium clusters (Nan) of 1000 atoms melts at 288 K, 10000 atoms melt at 303 K and bulk sodium melts at 371 K.
(5) Mechanical strength : The mechanic al strength of nano clusters increase the hardness of the metal.
For example : nanoparticles of copper and palladium clusters with diameter in the range of 5-7 nm have hardness up to 500 r. greater than the bulk metal.
(6) Electrical conductivity : At nanoscale level the electrical conductivity changes. For example : Carbon nanotubes behave as a conductor or semiconductor whereas carbon is nonconductor.
![]()
Question 15.
Describe the two methods of synthesising nanomaterials (nanoparticles).
Answer:
The two methods of synthesising nanomaterials :
(1) Bottom-up and
(2) Top down methods :
(1) Bottom-up method : Synthesis of nanoparticles in the bottom-up approach molecular components arrange themselves into more complex assemblies atom by atom, molecule by molecule and cluster by cluster from the bottom. Example : synthesis of nanoparticles by colloidal dispersion.
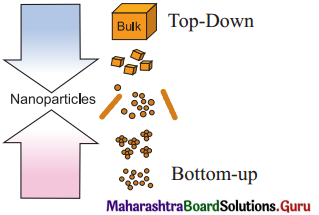
(2) Top-down method : In the top-down approach, involves nanomaterials being synthesised from bulk material by breaking the material. The bulk solids are disassembled into finer pieces until they are constituted of only few atoms. Example : Nanoparticles are synthesised by colloidal dispersion.
Question 16.
Discuss the various analytical tools used for characterization of nanoparticles.
Answer:
The analytical tools used for characterization of nanoparticles are
- U.V visible spectroscopy – It gives the preliminary confirmation of formation of nanoparticles.
- X-ray Diffraction (XRD) – The information given by this tool is about particle size, crystal structure and geometry.
- Scanning electron microscopy (SEM) : This is used to study the structure of surface of material that is the morphology of the material.
- Transmission electron microscopy (TEM) gives information about the particles size.
- (FTIR) Fourier transform infrared spectroscopy gives information about absorption of functional groups and binding nature of the nanomaterial.
Question 17.
Give evidence of use of nanoparticles by humans in ancient times with appropriate examples.
Answer:
There is enough evidence that nanomaterials have been produced and used by humans in ancient times. For example :
- Gold and silver nanoparticles trapped in the glass matrix gives ruby red colour in some ancient glass paintings.
- The decorative glaze or metallic film known as lustre found on some medieval pottery is due to certain spherical metallic nanoparticles.
- Carbon black is a nanostructured material that is used in tyres of car to increase the life of tyre. (Discovery in 1900). Carbon nanotubes are made up of graphite sheets with nanosized diameter. They have highest strength.
- Fumed silica, a component of silicon rubber, coatings, sealants and adhesives is also a nanostructured material.
Question 18.
Explain the different applications of nanoparticles.
Answer:
The contribution of nanochemistry in number of innovative products in various disciplines due to their unique physical, chemical, optical, structural, catalytic properties. Few applications are as follows :
- Nanoparticles contribute to stronger, lighter, cleaner and smarter surfaces and systems. They are used in the manufacture of scratchproof eyeglasses, transport, sunscreen, crack resistant paints, etc.
- Used in electronic devices like Magnetoresistive Random Access Memory (MRAM).
- Nanotechnology plays an important role in water purification techniques. Silver nanoparticles are used in water purification system to get safe drinking water.
- Self cleansing materials : Lotus is an example of self cleansing. Nanostructures on lotus leaves repel water which carries dirt as it rolls off. Lotus effect is the basis of self cleaning windows.
![]()
Question 19.
State the advantages of nanoparticles and nanotechnology.
Answer:
- Nanotechnology has revolutionalized electronics and computing.
- Nanotechnology has benefited the energy sector by making solar power more economical and energy storage more efficient.
- Nanotechnology has transformed the medical field with the manufacture of smart dmgs which help cure the life threatening diseases like cancer and diabetes faster and without side effects.
Question 20.
State the disadvantages of nanoparticles and nanotechnology.
Answer:
Despite the benefits that nanotechnology offers to the world, it is accompanied by certain disadvantages and potential risks.
The standard of living has been raised by nanotechnology but at the same time it has increased the environmental pollution. The kind of pollution caused by nanotechnology is very dangerous for living organism, it is called nano pollution.
Nanoparticles can be potential health hazard depending on the size, chemical composition and shape. They can be inhaled and can be deposited in the human respiratory tract and in the lungs, causing lung damage.
Question 21.
Name the development that meets the needs of the present, without compromising the ability of future generation to meet their own need.
Answer:
Sustainable development
Question 22.
Give name of father of green chemistry.
Answer:
Paul T. Anastas
Question 23.
Environmentally safe chemistry is known as.
Answer:
Green chemistry
Question 24.
How many principles does green chemistry have ?
Answer:
Twelve
Question 25.
Which principle of green chemistry has its perspective largely towards petrochemicals?
Answer:
Use of renewable feedstocks.
Question 26.
Name the chemical which leachs out of plastic packaging materials.
Answer:
Phthalate
Question 27.
Name the materials having structural components with at least one dimension in the nanometer scale.
Answer:
Nanomaterials.
Question 28.
Name the class of nanomaterial i.e. nanotubes, fibres, nanowires belong to.
Answer:
Two dimensions are in the nanoscale.
Question 29.
Name the nanoparticles used in sunscreen.
Answer:
Zinc oxide (ZnO) and Titanium dioxide (TiO2).
Question 30.
What is the colour of gold nanoparticles ?
Answer:
Red
Question 31.
Name the nanoparticles used as catalyst in hydrogenation reaction.
Answer:
Palladium and Platinum.
Question 32.
Name the two approaches used to synthesize nanomaterials.
Answer:
Bottom up and Top down.
Question 33.
Give the name of the wet chemical synthetic process for nanomaterials.
Answer:
Sol-gel process.
![]()
Question 34.
Give the steps involved in preparation of nanoparticle using sol-gel process.
Answer:
Hydrolysis, polycondensation, drying, thermal decomposition.
Question 35.
Name the analytical techniques used for characterisation of nanomaterials.
Answer:
u.v-visible spectroscopy, x-ray diffraction (XRD), scanning electron microscopy (SEM), Transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR).
Question 36.
Name the technique used to analyse particle size, crystal structure and geometry of a nanoparticle.
Answer:
x-ray diffraction (XRD)
Question 37.
Name the analytical technique used to study the morphology (structure of surface) of a material.
Answer:
Scanning electron microscopy (SEM)
Question 38.
Which innovative material has been developed using the lotus effect ?
Answer:
Self cleansing material
Question 39.
Which are the sectors that are revolutionalized by nanoparticles ?
Answer:
Electronics, energy sector and medical fields.
Question 40.
What are the disadvantages of nanotechnology ?
Answer:
Nano pollution and lung damage.
Question 41.
Name the scientist who coined the word nanotechnology.
Answer:
Nario Taniguchi (University professor at Tokyo in 1974).
Question 42.
Select and write the most appropriate answer from the given alternatives for each subquestion:
1. The measure of the amount of atoms from the starting materials that are present in the useful product at the end of chemical process is known as
(a) catalyst
(b) atom economy
(c) design of safer chemicals
(d) design for efficient energy
Answer:
(b) atom economy
2. The atom economy of the following reaction is CH3 – CH2 – CH2 – CH2 – OH + NaBr + H2SO4 → CH3 – CH2 – CH2 – CH2 – Br + NaHSO4 + H2O
(a) 49.81%
(b) 49%
(c) 50%
(d) 100%
Answer:
(a) 49.81%
3. Green chemistry reduces risk by
(a) developing the process for reuse and recycle of solvents and chemicals
(b) inventing technologies to clean the environ-ment
(c) minimize the use of chemicals
(d) reducing or eliminating the use or generation of hazardous chemicals in chemical products and process
Answer:
(d) reducing or eliminating the use or generation of hazardous chemicals in chemical products and process
4. Chemical synthesis should be designed to mini-mizes the use of
(a) liquid fuels
(b) solid fuels
(c) gaseous fuels
(d) energy
Answer:
(d) energy
5. The chemistry that applies across the life cycle of a chemical product like design, manufacture and use is called
(a) eco-friendly chemistry
(b) green chemistry
(c) environmental chemistry
(d) inorganic chemistry
Answer:
(b) green chemistry
![]()
6. According to the principles of green chemistry the chemicals involved in the production must be
(a) non-hazardous
(b) toxic
(c) polluting
(d) highly toxic
Answer:
(a) non-hazardous
7. Which of the following is not one of the twelve principles of green chemistry ?
(a) using renewable feedstocks
(b) designing safer chemicals and products
(c) maximizing atom economy
(d) avoiding the use of catalysts
Answer:
(d) avoiding the use of catalysts
8. Chemical synthesis based on principle of green chemistry encourages the use of
(a) hazardous chemicals
(b) reactions with low atom efficiency
(c) catalyst
(d) high energy requirements
Answer:
(c) catalyst
9. The plastic bottles made of HDPE are used to store household cleaner and shampoo can be recycled to make
(a) carpets, furniture, new containers
(b) detergent bottles, fencing, floor tiles, pens
(c) custom-made products
(d) cables, mudflaps, panelling, roadway gutters
Answer:
(b) detergent bottles, fencing, floor tiles, pens
10. The plastic ketch-up bottles and syrup bottles made from polypropylene (pp) can be recycled to make
(a) battery cables, brooms, ice scrapers, rakes
(b) envelopes, floor tiles, lumber
(c) custom-made products
(d) carpet, furniture, new containers
Answer:
(a) battery cables, brooms, ice scrapers, rakes
11. The role of green chemistry aims to
(a) design chemical processes and products that maximize profits
(b) design safer chemicals and products by reduc¬ing or eliminating the use and generation of hazardous substances
(c) design processes and products that work efficiently
(d) utilize non-renewable feedstocks
Answer:
(b) design safer chemicals and products by reducing or eliminating the use and generation of hazardous substances
12. The study of phenomena and manipulation of materials of atomic, molecular and macromolecular scales where properties differ significantly from those at a large scale is called
(a) nanoscience
(b) nanochemistry
(c) nanotechnology
(d) nanomaterial
Answer:
(a) nanoscience
13. The term nanotechnology was first used by whom and when ?
(a) Richard Feynman, 1959
(b) Nario Taniguchi, 1974
(c) Eric Drexter, 1986
(d) Sumia Lijima, 1991
Answer:
(b) Nario Taniguchi, 1974
14. Which one of these statements is NOT true ?
(a) Gold at the nanoscale is red.
(b) A very highly useful application of nanochem¬istry is medicine.
(c) Sunscreen contains nanoparticles of zinc oxide (ZnO) and (SiO2) silicon oxide.
(d) Silicon at nanoscale is not an insulator
Answer:
(c) Sunscreen contains nanoparticles of zinc oxide (ZnO) and (SiO2) silicon oxide.
![]()
15. Which of the historical works mentioned below contain nanotechnology?
(a) Lycurgus cup
(b) Medieval stained glass windows in churches
(c) Damascus steel swords
(d) All of the above
Answer:
(d) All of the above
16. The nanometer scale is conventionally defined as
(a) 10 – 100nm
(b) 1 – 100nm
(c) 1 – 1000 nm
(d) 1 – 10000 nm
Answer:
(b) 1-100 nm
17. The material synthesized on the nanometer scale possess
(a) same bulk properties
(b) different bulk properties
(c) unique optical, magnetic, electrical properties
(d) no change in properties
Answer:
(c) unique optical, magnetic, electrical properties
18. Nanomaterials of zero dimension is
(a) one in which all three dimensions are in the nanoscale
(b) one in which two dimensions are in the nanoscale
(c) one in which one dimension is in the nanoscale
(d) None of the above
Answer:
(a) one in which all three dimensions are in the nanoscale
19. The science which deals with the design and synthesis of material on nanoscale with different size and shape is called
(a) nanoscience
(b) nanochemistry
(c) nanophysics
(d) nanotechnology
Answer:
(b) nanochemistry
20. Elemental has a shining yellow colour, but the colour of nanoparticles of gold is
(a) green
(b) yellow
(c) red
(d) blue
Answer:
(c) red
21. The surface area of nanoparticles
(a) is the same as in bulk
(b) increases with the same volume of the bulk
(c) decreases with the same volume of the bulk
(d) does not change with particle size
Answer:
(b) increases with the same volume of the bulk
22. The nanomaterial based catalyst are usually
(a) homogeneous catalyst
(b) heterogeneous catalyst
(c) good catalyst
(d) bad catalyst
Answer:
(b) heterogeneous catalyst
23. The catalyst used in photocatalysis is
(a) gold
(b) Raney Ni
(c) TiO2
(d) AI2O3
Answer:
(c) TiO2
![]()
24. Nanosized copper clusters have the mechanical strength of
(a) less than the bulk copper wire
(b) 100% more than the bulk metal
(c) 200% more than the bulk metal
(d) 500% more than the bulk metal
Answer:
(d) 500% more than the bulk metal
25. The most common method used for synthesis of nanomaterials is
(a) sol-gels method
(b) only sol method
(c) only gel method
(d) colloidal dispersion method
Answer:
(a) sol-gels method
26. What is the information obtained from uv-visible spectroscopy when used for nanomaterials ?
(a) morphology of structure
(b) preliminary conformation of formation of nanoparticle
(c) particle size
(d) functional group present
Answer:
(b) preliminary conformation of formation of nanoparticle
27. What information of the nanoparticles is obtained from transmission electron microscopy technique ?
(a) structure
(b) functional group
(c) particle size
(d) geometry
Answer:
(c) particle size
28. The analytical tool used to study the structure of surface of nanoparticle i.e. morphology is
(a) Absorption spectroscopy
(b) Scanning electron microscopy
(c) Emission spectroscopy
(d) Nuclear magnetic resonance spectroscopy
Answer:
(b) Scanning electron microscopy
29. The constituents of carbon nanotubes are
(a) nanosized graphite sheets
(b) nanosized carbon black
(c) nanosized coal black
(d) None of the above
Answer:
(a) nanosized graphite sheets
30. Self cleansing windows are example of the
(a) Nanoparticle effect
(b) Crompton effect
(c) Lotus effect
(d) Tyndal effect
Answer:
(c) Lotus effect
![]()
31. Which highly effective and cost effective nano-particles are used for water purification ?
(a) gold nanoparticle
(b) copper nanoparticle
(c) silver nanoparticle
(d) silica nanoparticle
Answer:
(c) silver nanoparticle
32. The sectors revolutionalized by nanotechnology are
(a) electronics and computing
(b) energy
(c) medicine
(d) All of the above
Answer:
(d) All of the above
33. Name the body part that gets affected by the hazardous nano pollution.
(a) heart
(b) brain
(c) lungs
(d) eyes
Answer:
(c) lungs
34. The pollution caused by nanotechnology is known as
(a) air pollution
(b) nano pollution
(c) ground pollution
(d) environmental pollution
Answer:
(b) nano pollution.